Abstract
Purpose
Comparability of patient-reported outcome measures over different languages is essential to allow cross-national research. We investigate the comparability of the PROMIS Profile 29, a generic health-related quality of life measure, in general population samples in the UK, France, and Germany and present general population reference values.
Methods
A web-based survey was simultaneously conducted in the UK (n = 1509), France (1501), and Germany (1502). Along with the PROMIS Profile 29, we collected sociodemographic information as well as the EQ-5D. We tested measurement invariance by means of multigroup confirmatory factor analysis (CFA). Differences in the health-related quality of life between countries were modeled by linear regression analysis. We present general population reference data for the included PROMIS domains utilizing plausible value imputation and quantile regression.
Results
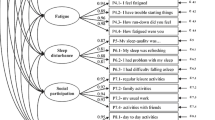
Multigroup CFA of the PROMIS Profile 29 showed that factor means are insensitive to potential measurement bias except in one item. We observed significant differences in patient-reported health between countries, which could be partially explained by the differences in overall ratings of health. The physical function and pain interference scales showed considerable floor effects in the normal population in all countries.
Conclusions
Scores derived from the PROMIS Profile 29 are largely comparable across the UK, France, and Germany. Due to the use of plausible value imputation, the presented general population reference values can be compared to data collected with other PROMIS short forms or computer-adaptive tests.


Similar content being viewed by others
References
Basch, E. (2017). Patient-reported outcomes—Harnessing patients’ voices to improve clinical care. New England Journal of Medicine, 376(2), 105–108. https://doi.org/10.1056/NEJMp1002530.
Snyder, C. F., Jensen, R. E., Segal, J. B., & Wu, A. W. (2013). Patient-reported outcomes (PROs) outcomes research. Medical Care, 51(8 Suppl 3), 73–79.
Black, N., Burke, L., Forrest, C. B., Sieberer, U. R., Ahmed, S., Valderas, J. M., … Alonso, J. (2016). Patient-reported outcomes: Pathways to better health, better services, and better societies. Quality of Life Research, 25(5), 1103–1112. https://doi.org/10.1007/s11136-015-1168-3.
McNamara, R. L., Spatz, E. S., Kelley, T. A., Stowell, C. J., Beltrame, J., Heidenreich, P., … Lewin, J. (2015). Standardized outcome measurement for patients with coronary artery disease: Consensus from the International Consortium for Health Outcomes Measurement (ICHOM). Journal of the American Heart Association, 4(5), e001767. https://doi.org/10.1161/JAHA.115.001767.
Ware, J. E., Kosinski, M., Gandek, B., & Aaronson, N. (1998). The factor structure of the SF-36 health survey in 10 countries: Results from the IQOLA project. Journal of Clinical Epidemiology, 51(11), 1159–1165.
Bullinger, M., Alonso, J., Apolone, G., Leplège, A., & Sullivan, M. (1998). Translating health status questionnaires and evaluating their quality: The IQOLA project approach. Journal of Clinical Epidemiology, 51(11), 913–923.
Szende, A., Janssen, B., & Cabases, J. (2014). Self-reported population health: An international perspective based on EQ-5D. Dordrecht: Springer.
Beaton, D. E., Bombardier, C., Guillemin, F., & Ferraz, M. B. (2000). Guidelines for the process of cross-cultural adaptation of self-report measures. Spine, 25(24), 3186–3191.
Alonso, J., Bartlett, S. J., Rose, M., Aaronson, N., Chaplin, J. E., Efficace, F., … Forrest, C. B. (2013). The case for an international patient-reported outcomes measurement information system (PROMIS) initiative. Health and Quality of Life Outcomes, 11(210), 1–5. https://doi.org/10.1186/1477-7525-11-210.
Cella, D., Yount, S., Rothrock, N., Gershon, R., Cook, K. F., Reeve, B. B., … Rose, M. (2007). Developing the patient-reported outcomes measurement information system (PROMIS). Medical Care, 45(5), 3–11. Retrieved from http://journals.lww.com/lww-medicalcare/Abstract/2007/05001/Developing_the_Patient_Reported_Outcomes.1.aspx.
Cella, D., Yount, S., Rothrock, N., & Gershon, R. (2007). The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Medical Care, 45(5), 3–11.
Reeve, B. B., Hays, R. D., Bjorner, J. B., Cook, K. F., Crane, P. K., Teresi, J. A., … Cella, D. (2007). Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Medical Care, 45(5 Suppl 1), S22–S31. https://doi.org/10.1097/01.mlr.0000250483.85507.04.
Böhnke, J. R., & Lutz, W. (2014). Using item and test information to optimize targeted assessments of psychological distress. Assessment, 21(6), 679–693. https://doi.org/10.1177/1073191114529152.
Patient-Reported Outcomes Measurement Information System. (2013). PROMIS instrument development and validation scientific standards version 2.0. Retrieved March 20, 2016, from http://www.nihpromis.org/Documents/PROMISStandards_Vers2.0_Final.pdf.
Choi, S. W., Schalet, B. D., Cook, K. F., & Cella, D. (2014). Establishing a common metric for depressive symptoms: Linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychological Assessment, 26(2), 513–527. https://doi.org/10.1037/a0035768.
Schalet, B. D., Cook, K. F., Choi, S. W., & Cella, D. (2014). Establishing a common metric for self-reported anxiety: Linking the MASQ, PANAS, and GAD-7 to PROMIS Anxiety. Journal of Anxiety Disorders, 28(1), 88–96. https://doi.org/10.1016/j.janxdis.2013.11.006.
Fischer, H. F., Tritt, K., Klapp, B. F., & Fliege, H. (2011). How to compare scores from different depression scales: Equating the Patient Health Questionnaire (PHQ) and the ICD-10-symptom rating (ISR) using item response. International Journal of Methods in Psychiatric Research, 20(4), 203–214. https://doi.org/10.1002/mpr.
Wahl, I., Löwe, B., Bjorner, J. B., Fischer, H. F., Langs, G., Voderholzer, U., … Rose, M. (2014). Standardization of depression measurement: A common metric was developed for 11 self-report depression measures. Journal of Clinical Epidemiology, 67(1), 73–86. https://doi.org/10.1016/j.jclinepi.2013.04.019.
Millsap, R. E. (2011). Statistical approaches to measurement invariance. New York: Routledge.
Holland, P., & Wainer, H. (2012). Differential item functioning. Hillsdale: Lawrence Erlbaum Associates, Inc.
Reeve, B. B., Wyrwich, K. W., Wu, A. W., Velikova, G., Terwee, C. B., Snyder, C. F., … Butt, Z. (2013). ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Quality of Life Research. https://doi.org/10.1007/s11136-012-0344-y.
Bartlett, S. J., Witter, J., Cella, D., & Ahmed, S. (2017). Montreal accord on patient-reported outcomes use series-paper 6: Creating national initiatives to support development and use-the PROMIS example. Journal of Clinical Epidemiology. https://doi.org/10.1016/j.jclinepi.2017.04.015.
Wahl, I., Löwe, B., & Rose, M. (2011). Das Patient-Reported Outcomes Measurement Information System (PROMIS): Übersetzung der Item-Banken für Depressivität und Angst ins Deutsche. Klinische Diagnostik und Evaluation, 4, 236–261.
Farin, E., Nagl, M., Gramm, L., Heyduck, K., & Glattacker, M. (2013). Development and evaluation of the PI-G: A three-scale measure based on the German translation of the PROMIS pain interference item bank. Quality of Life Research. https://doi.org/10.1007/s11136-013-0575-6.
Liegl, G., Rose, M., Correia, H., Fischer, H. F., Kanlidere, S., Mierke, A., … Nolte, S. (2017). An initial psychometric evaluation of the German PROMIS v1.2 physical function item bank in patients with a wide range of health conditions. Clinical Rehabilitation, 26921551771429. https://doi.org/10.1177/0269215517714297.
Paz, S. H., Spritzer, K. L., Morales, L. S., & Hays, R. D. (2013). Evaluation of the Patient-Reported Outcomes Information System (PROMIS(®)) Spanish-language physical functioning items. Quality of Life Research, 22(7), 1819–1830. https://doi.org/10.1007/s11136-012-0292-6.
Oude Voshaar, M. A. H., ten Klooster, P. M., Glas, C., Vonkeman, H. E., Taal, E., Krishnan, E., … van de Laar, M. A. (2014). Calibration of the promis physical function item bank in Dutch patients with rheumatoid arthritis. PLoS ONE, 9(3), e92367. https://doi.org/10.1371/journal.pone.0092367.
Crins, M. H. P., Roorda, L. D., Smits, N., de Vet, H. C. W., Westhovens, R., Cella, D., … Terwee, C. B. (2015). Calibration and validation of the Dutch-flemish PROMIS pain interference item bank in patients with chronic pain. PLoS ONE, 10(7), e0134094. https://doi.org/10.1371/journal.pone.0134094.
Fischer, H. F., Wahl, I., Nolte, S., Liegl, G., Brähler, E., Löwe, B., & Rose, M. (2016). Language-related differential item functioning between English and German PROMIS depression items is negligible. International Journal of Methods in Psychiatric Research, 26(4), e1530. https://doi.org/10.1002/mpr.1530.
Hahn, E. A., DeWalt, D. A., Bode, R. K., Garcia, S. F., Devellis, R. F., Correia, H., & Cella, D. (2014). New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 33(5), 490–499. https://doi.org/10.1037/hea0000055.
Choi, S. W., Reise, S. P., Pilkonis, P. A., Hays, R. D., & Cella, D. (2010). Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Quality of Life Research, 19(1), 125–136. https://doi.org/10.1007/s11136-009-9560-5.
Cella, D., Gershon, R., Lai, J.-S., Choi, S. W., Yount, S., Rothrock, N., … Rose, M. (2007). The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Quality of Life Research, 16(5 Suppl 1), 133–141. https://doi.org/10.1007/s11136-007-9204-6.
van Reenen, M., & Janssen, B. (2015). EQ-5D-5L user guide. Basic information on how to use the EQ-5D-5L instrument. Retrieved from https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf. Accessed on 16 Jan 2018.
Valderas, J. M., & Alonso, J. (2008). Patient reported outcome measures: A model-based classification system for research and clinical practice. Quality of Life Research, 17(9), 1125–1135. https://doi.org/10.1007/s11136-008-9396-4.
Patient-Reported Outcomes Measurement Information System. (2013). PROMIS short form scoring manual. Retrieved March 21, 2016, from http://www.assessmentcenter.net/documents/PROMIS%20Profile%20Scoring%20Manual.pdf.
Amtmann, D., Cook, K. F., Jensen, M. P., Chen, W., Choi, S., Revicki, D. A., … Callahan, L. (2010). Development of a PROMIS item bank to measure pain interference. Pain, 150(1), 173–182. https://doi.org/10.1016/j.pain.2010.04.025.Development.
Hahn, E. A., Devellis, R. F., Bode, R. K., Garcia, S. F., Castel, L. D., Eisen, S. V., …Cella, D. (2010). Measuring social health in the patient-reported outcomes measurement information system (PROMIS): Item bank development and testing. Quality of Life Research, 19(7), 1035–1044. https://doi.org/10.1007/s11136-010-9654-0.
Lai, J. S., Cella, D., Choi, S., Junghaenel, D. U., Christodoulou, C., Gershon, R., & Stone, A. (2011). How item banks and their application can influence measurement practice in rehabilitation medicine: A PROMIS fatigue item bank example. Archives of Physical Medicine and Rehabilitation, 92(10 Suppl.), S20–S27. https://doi.org/10.1016/j.apmr.2010.08.033.
Pilkonis, P. A., Choi, S. W., Reise, S. P., Stover, A. M., Riley, W. T., & Cella, D. (2011). Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment, 18(3), 263–283. https://doi.org/10.1177/1073191111411667.
Rose, M., Bjorner, J. B., Gandek, B., Bruce, B., Fries, J. F., & Ware, J. E. (2014). The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. Journal of Clinical Epidemiology, 67(5), 516–526. https://doi.org/10.1016/j.jclinepi.2013.10.024.
Buysse, D. J., Yu, L., Moul, D. E., Germain, A., Stover, A., Dodds, N. E., … Pilkonis, P. A. (2010). Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep, 33(6), 781–92.
Schalet, B. D., Hays, R. D., Jensen, S. E., Beaumont, J. L., Fries, J. F., & Cella, D. (2016). Validity of PROMIS physical function measures in diverse clinical samples. Journal of Clinical Epidemiology, 73, 112–118. https://doi.org/10.1016/j.jclinepi.2015.08.039.
Schalet, B. D., Pilkonis, P. A., Yu, L., Dodds, N., Johnston, K. L., Yount, S., … Cella, D. (2016). Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. Journal of Clinical Epidemiology, 73, 119–127. https://doi.org/10.1016/j.jclinepi.2015.08.036.
Cella, D., Lai, J.-S., Jensen, S. E., Christodoulou, C., Junghaenel, D. U., Reeve, B. B., & Stone, A. A. (2016). Clinical validity of the PROMIS® fatigue item bank across diverse clinical samples. Journal of Clinical Epidemiology, 73, 128–134. https://doi.org/10.1016/j.jclinepi.2015.08.037.
Cook, K. F., Jensen, S. E., Schalet, B. D., Beaumont, J. L., Amtmann, D., Czajkowski, S., … Cella, D. (2016). PROMIS® measures of pain, fatigue, negative affect, physical function and social function demonstrate clinical validity across a range of chronic conditions. Journal of Clinical Epidemiology, 73, 89–102. https://doi.org/10.1016/j.jclinepi.2015.08.038.
Hahn, E. A., Beaumont, J. L., Pilkonis, P. A., Garcia, S. F., Magasi, S., DeWalt, D. A., & Cella, D. (2016). The PROMIS satisfaction with social participation measures demonstrate responsiveness in diverse clinical populations. Journal of Clinical Epidemiology, 73, 135–141. https://doi.org/10.1016/j.jclinepi.2015.08.034.
Stone, A. A., Broderick, J. E., Junghaenel, D. U., Schneider, S., & Schwartz, J. E. (2015). PROMIS fatigue, pain intensity, pain interference, pain behavior, physical function, depression, anxiety, and anger scales demonstrate ecological validity. Journal of Clinical Epidemiology, 74, 194–206. https://doi.org/10.1016/j.jclinepi.2015.08.029.
Askew, R. L., Cook, K. F., Revicki, D. A., Cella, D., & Amtmann, D. (2016). Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. Journal of Clinical Epidemiology, 73, 103–111. https://doi.org/10.1016/j.jclinepi.2015.08.035.
Beaumont, J. L., Cella, D., Phan, A. T., Choi, S., Liu, Z., & Yao, J. C. (2012). Comparison of health-related quality of life in patients with neuroendocrine tumors with quality of life in the general US population. Pancreas, 41(3), 461–466. https://doi.org/10.1097/MPA.0b013e3182328045.
Craig, B. M., Reeve, B. B., Brown, P. M., Cella, D., Hays, R. D., Lipscomb, J., … Revicki, D. A. (2014). US valuation of health outcomes measured using the PROMIS-29. Value in Health, 17(8), 846–853. https://doi.org/10.1016/j.jval.2014.09.005.
Pearman, T. P., Beaumont, J. L., Cella, D., Neary, M. P., & Yao, J. (2016). Health-related quality of life in patients with neuroendocrine tumors: An investigation of treatment type, disease status, and symptom burden. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. https://doi.org/10.1007/s00520-016-3189-z.
Yount, S. E., Beaumont, J. L., Chen, S.-Y., Kaiser, K., Wortman, K., Van Brunt, D. L., … Cella, D. (2016). Health-related quality of life in patients with idiopathic pulmonary fibrosis. Lung, 194(2), 227–234. https://doi.org/10.1007/s00408-016-9850-y.
Hinchcliff, M., Beaumont, J. L., Thavarajah, K., Varga, J., Chung, A., Podlusky, S., … Cella, D. (2011). Validity of two new patient-reported outcome measures in systemic sclerosis: Patient-reported outcomes measurement information system 29-item health profile and functional assessment of chronic illness therapy-dyspnea short form. Arthritis Care & Research, 63(11), 1620–1628. https://doi.org/10.1002/acr.20591.
Hinchcliff, M., Beaumont, J. L., Carns, M., Podlusky, S., Thavarajah, K., Varga, J., … Chang, R. W. (2015). Longitudinal evaluation of PROMIS-29 and FACIT-dyspnea short forms in systemic sclerosis. Journal of Rheumatology, 42(1), 64–72. https://doi.org/10.1530/ERC-14-0411.Persistent.
Kirwan, J. R., & Reeback, J. S. (1986). Using a modified Stanford Health Assessment Questionnaire to access disability in UK patients with rheumatoid arthritis. British Journal of Rheumatology, 25, 206–209. https://doi.org/10.1093/rheumatology/25.2.206.
Muthén, L. K., & Muthén, B. (n.d.). Mplus user’s guide. Los Angeles: Muthén & Muthén. https://doi.org/10.1111/j.1600-0447.2011.01711.x.
Muthén, B. O., & Asparouhov, T. (2002). Latent variable analysis with categorical outcomes: Multiple-group and growth modeling in Mplus. Mplus Web Notes, 4.
Wu, H., & Estabrook, R. (2016). Identification of confirmatory factor analysis models of different levels of invariance for ordered categorical outcomes. Psychometrika, 81(4), 1014–1045. https://doi.org/10.1007/s11336-016-9506-0.
Millsap, R. E., & Tein, J. Y. (2004). Multivariate behavioral assessing factorial invariance in ordered-categorical measures. Multivariate Behavioral Research, 39(3), 479–515. https://doi.org/10.1207/S15327906MBR3903.
Brown, T. A., & Kenny, D. A. (2006). Confirmatory factor analysis for applied research. New York: Guilford Press.
Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. https://doi.org/10.1080/10705519909540118.
Asparouhov, T., & Muthen, B. (2006). Robust chi square difference testing with mean and variance adjusted test statistics. Mplus Web Notes, 10, 1–6. Retrieved from http://www.statmodel.com/download/webnotes/webnote10.pdf.
Davidov, E., Meuleman, B., Cieciuch, J., Schmidt, P., & Billiet, J. (2014). Measurement equivalence in cross-national research. Annual Review of Sociology, 40(1), 55–75. https://doi.org/10.1146/annurev-soc-071913-043137.
Chen, F. F. (2007). Sensitivity of goodness of fit indexes to lack of measurement invariance. Structural Equation Modeling: A Multidisciplinary Journal, 14(3), 464–504. https://doi.org/10.1080/10705510701301834.
Meade, A. W., Johnson, E. C., & Braddy, P. W. (2008). Power and sensitivity of alternative fit indices in tests of measurement invariance. Journal of Applied Psychology, 93(3), 568–592. https://doi.org/10.1037/0021-9010.93.3.568.
Sass, D. A., Schmitt, T. A., & Marsh, H. W. (2014). Evaluating model fit with ordered categorical data within a measurement invariance framework: A comparison of estimators. Structural Equation Modeling: A Multidisciplinary Journal, 21(2), 167–180. https://doi.org/10.1080/10705511.2014.882658.
Glas, C., Geerlings, H., van de Laar, M. A. F. J., & Taal, E. (2009). Analysis of longitudinal randomized clinical trials using item response models. Contemporary Clinical Trials, 30(2), 158–170. https://doi.org/10.1016/j.cct.2008.12.003.
Gorter, R., Fox, J.-P., & Twisk, J. (2015). Why item response theory should be used for longitudinal questionnaire data analysis in medical research. BMC Medical Research Methodology, 2, 1–12. https://doi.org/10.1186/s12874-015-0050-x.
Levy, R., & Mislevy, R. J. (2016). Bayesian psychometric modeling. Boca Raton: CRC Press.
Marshall, A., Altman, D. G., Holder, R. L., & Royston, P. (2009). Combining estimates of interest in prognostic modelling studies after multiple imputation: Current practice and guidelines. BMC Medical Research Methodology, 9, 57. https://doi.org/10.1186/1471-2288-9-57.
Hao, L., & Naiman, D. Q. (2007). Quantile regression. Thousand Oaks: Sage.
R Development Core Team. (2008). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Pasek, J. (2016). weights: Weighting and weighted statistics. R package. Retrieved from http://cran.r-project.org/package=weights. Accessed 16 Jan 2018.
Koenker, R. (2016). quantreg: Quantile regression. R package. Retrieved from http://cran.r-project.org/package=quantreg. Accessed 16 Jan 2018.
Honaker, J., King, G., & Blackwell, M. (2011). AMELIA II: A program for missing data. Journal of Statistical Software, 45(7), 1–54.
Janssen, B., & Szende, A. (2014). Population norms for the EQ-5D. In A. Szende, B. Janssen & J. Cabases (Eds.), Self-reported population health: An international perspective based on EQ-5D (pp. 19–30). Dordrecht: Springer.
Chalmers, R. P., Counsell, A., & Flora, D. B. (2016). It might not make a big DIF. Educational and Psychological Measurement, 76(1), 114–140. https://doi.org/10.1177/0013164415584576.
Katz, P., Pedro, S., & Michaud, K. (2016). Performance of the PROMIS 29-item profile in rheumatoid arthritis, osteoarthritis, fibromyalgia, and systemic lupus erythematosus. Arthritis Care & Research. https://doi.org/10.1002/acr.
Marsman, M., Maris, G., Bechger, T., & Glas, C. (2016). What can we learn from Plausible Values? Psychometrika. https://doi.org/10.1007/s11336-016-9497-x.
Cella, D., Riley, W., Stone, A., Northrock, N., Reeve, B. B., Yount, S., … Hays, R. D. (2010). Initial adult health item banks and first wave testing of the patient-reported outcomes measurement information system (PROMIS™) network: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. https://doi.org/10.1016/j.jclinepi.2010.04.011.Initial.
Gorter, R., Fox, J.-P., Apeldoorn, A., & Twisk, J. (2016). The influence of measurement model choice for randomized controlled trial results. Journal of Clinical Epidemiology. https://doi.org/10.1016/j.jclinepi.2016.06.011.
Thissen, D., & Wainer, H. (2001). Test scoring. Mahwah, NJ: Lawrence Erlbaum Associates.
Liegl, G., Wahl, I., Berghöfer, A., Nolte, S., Pieh, C., Rose, M., & Fischer, H. F. (2016). Using PHQ-9 item parameters of a common metric resulted in similar depression scores compared to independent IRT model reestimation. Journal of Clinical Epidemiology, 71, 25–34. https://doi.org/10.1016/j.jclinepi.2015.10.006.
Acknowledgements
This study was funded by the Centre Virchow-Villerme (https://virchowvillerme.eu/). We like to acknowledge the many people involved in development and translation of the PROMIS measures used in this study. Our thanks for their efforts to translate various PROMIS measures into German and French go in particular to Susan Bartlett, Marie-Eve Carrier, Erik Farin-Glattacker, Katja Heyduck, Sandra Nolte, and Inka Wahl. We thank Laurence Erdur and Nina Obbarius for their help in comparing the different language versions and Terrence Jorgensen for illuminating the pitfalls in measurement invariance testing of ordinal data. Furthermore, we would like to address special thanks to PROMIS translation manager Helena Correia.
Funding
This study was funded by the Centre Virchow-Villerme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fischer, F., Gibbons, C., Coste, J. et al. Measurement invariance and general population reference values of the PROMIS Profile 29 in the UK, France, and Germany. Qual Life Res 27, 999–1014 (2018). https://doi.org/10.1007/s11136-018-1785-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-018-1785-8