Abstract
Purpose
The primary objective is to provide evidence of convergent and discriminant validity for the pediatric and parent-proxy versions of the Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety, Depression, Anger, Peer Relations, Mobility, Pain Interference, and Fatigue item banks, the Neurology Quality of Life measurement system (Neuro-QOL) Cognition-General Concerns and Stigma item banks, and the Traumatic Brain Injury Quality of Life (TBI-QOL) Executive Function and Headache item banks in a pediatric traumatic brain injury (TBI) sample.
Methods
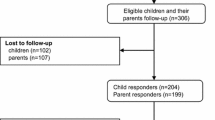
Participants were 134 parent–child (ages 8–18 years) days. Children all sustained TBI and the dyads completed outcome ratings 6 months after injury at one of six medical centers across the United States. Ratings included PROMIS, Neuro-QOL, and TBI-QOL item banks, as well as the Pediatric Quality of Life inventory (PedsQL), the Health Behavior Inventory (HBI), and the Strengths and Difficulties Questionnaire (SDQ) as legacy criterion measures against which these item banks were validated.
Results
The PROMIS, Neuro-QOL, and TBI-QOL item banks demonstrated good convergent validity, as evidenced by moderate to strong correlations with comparable scales on the legacy measures. PROMIS, Neuro-QOL, and TBI-QOL item banks showed weaker correlations with ratings of unrelated constructs on legacy measures, providing evidence of discriminant validity.
Conclusion
Our results indicate that the constructs measured by the PROMIS, Neuro-QOL, and TBI-QOL item banks are valid in our pediatric TBI sample and that it is appropriate to use these standardized scores for our primary study analyses.
Similar content being viewed by others
Abbreviations
- CAT:
-
Computer Adaptive Test
- CDC:
-
Centers for Disease Control and Prevention
- GCS:
-
Glasgow Coma Scale
- HRQOL:
-
Health-related quality of life
- IRB:
-
Institutional Review Board
- IRT:
-
Item response theory
- NEURO-QOL:
-
Neurology Quality of Life (measurement system)
- PedsQL:
-
Pediatric Quality of Life (outcome measure)
- PRO:
-
Patient-reported outcomes
- PROMIS:
-
Patient-Reported Outcomes Measurement Information System
- SF:
-
Short form
- TBI:
-
Traumatic brain injury
- TBI-QOL:
-
Traumatic Brain Injury Quality of Life
References
Rivara, F. P., Koepsell, T. D., Wang, J., et al. (2012). Incidence of disability among children 12 months after traumatic brain injury. American Journal of Public Health, 102(11), 2074–2079.
Rivara, F. P., Vavilala, M. S., Durbin, D., et al. (2012). Persistence of disability 24 to 36 months after pediatric traumatic brain injury: A cohort study. Journal of Neurotrauma, 29(15), 2499–2504.
Rivara, F. P., Koepsell, T. D., Wang, J., et al. (2011). Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics, 128(5), e1129–e1138.
Stanley, R. M., Bonsu, B. K., Zhao, W., Ehrlich, P. F., Rogers, A. J., & Xiang, H. (2012). US estimates of hospitalized children with severe traumatic brain injury: Implications for clinical trials. Pediatrics, 129(1), e24–e30.
Cella, D., Yount, S., Rothrock, N., et al. (2007). The patient-reported outcomes measurement information system (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Medical Care, 45(5 Suppl 1), S3–S11.
Fries, J. F., Bruce, B., & Cella, D. (2005). The promise of PROMIS: Using item response theory to improve assessment of patient-reported outcomes. Clinical and Experimental Rheumatology, 23(5), 53–57.
Whyte, J., Vasterling, J., & Manley, G. T. (2010). Common data elements for research on traumatic brain injury and psychological health: Current status and future development. Archives of Physical Medicine and Rehabilitation, 91(11), 1692–1696.
Wilde, E. A., Whiteneck, G. G., Bogner, J., et al. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Archives of Physical Medicine and Rehabilitation, 91(11), 1650–1660.
DeWalt, D. A., Rothrock, N., Yount, S., & Stone, A. A. (2007). Evaluation of item candidates: The PROMIS qualitative item review. Medical Care, 45(5 Suppl 1), S12–S21.
Gershon, R. C., Rothrock, N., Hanrahan, R., Bass, M., & Cella, D. (2010). The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. Journal of Applied Measurement, 11(3), 304–314.
Carle, A. C., Cella, D., Cai, L., et al. (2011). Advancing PROMIS’s methodology: Results of the third patient-reported outcomes measurement information system (PROMIS(R)) Psychometric Summit. Expert review of pharmacoeconomics & outcomes. Research, 11(6), 677–684.
Broderick, J. E., DeWitt, E. M., Rothrock, N., Crane, P. K., & Forrest, C. B. (2013). Advances in patient-reported outcomes: The NIH PROMIS((R)) measures. EGEMS (Washington, DC), 1(1), 1015.
Cella, D., Riley, W., Stone, A., et al. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194.
Schalet, B. D., Revicki, D. A., Cook, K. F., Krishnan, E., Fries, J. F., & Cella, D. (2015). Establishing a common metric for physical function: Linking the HAQ-DI and SF-36 PF subscale to PROMIS((R)) physical function. Journal of General Internal Medicine, 30(10), 1517–1523.
Irwin, D. E., Stucky, B., Langer, M. M., et al. (2010). An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Quality of Life Research, 19(4), 595–607.
Irwin, D. E., Stucky, B. D., Langer, M. M., et al. (2012). PROMIS pediatric anger scale: An item response theory analysis. Quality of Life Research, 21(4), 697–706.
Bevans, K. B., Gardner, W., Pajer, K., Riley, A. W., & Forrest, C. B. (2013). Qualitative development of the PROMIS(R) pediatric stress response item banks. Journal of Pediatric Psychology, 38(2), 173–191.
Reeve BB, Thissen D, DeWalt DA, et al. Linkage between the PROMIS pediatric and adult emotional distress measures. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2015.
Dewalt, D. A., Thissen, D., Stucky, B. D., et al. (2013). PROMIS Pediatric Peer Relationships Scale: Development of a peer relationships item bank as part of social health measurement. Health Psychology : Official Journal of the Division of Health Psychology, American Psychological Association, 32(10), 1093–1103.
Lai, J. S., Stucky, B. D., Thissen, D., et al. (2013). Development and psychometric properties of the PROMIS((R)) pediatric fatigue item banks. Quality of Life Research. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 22(9), 2417–2427.
Varni, J. W., Stucky, B. D., Thissen, D., et al. (2010). PROMIS pediatric pain interference scale: An item response theory analysis of the pediatric pain item bank. Journal of Pain, 11(11), 1109–1119.
Jacobson, C. J. Jr., Kashikar-Zuck, S., Farrell, J., et al. (2015). Qualitative evaluation of pediatric pain behavior, quality, and intensity item candidates and the PROMIS pain domain framework in children with chronic pain. The Journal of Pain : Official Journal of The American Pain Society, 16(12), 1243–1255.
Jacobson, C. J., Farrell, J. E., Kashikar-Zuck, S., Seid, M., Verkamp, E., & Dewitt, E. M. (2013). Disclosure and self-report of emotional, social, and physical health in children and adolescents with chronic pain—a qualitative study of PROMIS pediatric measures. Journal of Pediatric Psychology, 38(1), 82–93.
Kashikar-Zuck S, Carle A, Barnett K, et al. Longitudinal evaluation of patient reported outcomes measurement information systems (PROMIS) measures in pediatric chronic pain. Pain. 2015.
Irwin, D. E., Stucky, B. D., Thissen, D., et al. (2010). Sampling plan and patient characteristics of the PROMIS pediatrics large-scale survey. Quality of Life Research, 19(4), 585–594.
Yeatts, K. B., Stucky, B., Thissen, D., et al. (2010). Construction of the pediatric asthma impact scale (PAIS) for the patient-reported outcomes measurement information system (PROMIS). Journal of Asthma, 47(3), 295–302.
Quinn, H., Thissen, D., Liu, Y., et al. (2014). Using item response theory to enrich and expand the PROMIS(R) pediatric self report banks. Health and Quality of Life Outcomes, 12, 160.
Ravens-Sieberer, U., Devine, J., Bevans, K., et al. (2014). Subjective well-being measures for children were developed within the PROMIS project: Presentation of first results. Journal of Clinical Epidemiology, 67(2), 207–218.
Tucker, C. A., Bevans, K. B., Teneralli, R. E., Smith, A. W., Bowles, H. R., & Forrest, C. B. (2014). Self-reported pediatric measures of physical activity, sedentary behavior, and strength impact for PROMIS: Conceptual framework. Pediatric Physical Therapy: The Official Publication of the Section on Pediatrics of the American Physical Therapy Association, 26(4), 376–384.
Tucker, C. A., Bevans, K. B., Teneralli, R. E., Smith, A. W., Bowles, H. R., & Forrest, C. B. (2014). Self-reported pediatric measures of physical activity, sedentary behavior, and strength impact for PROMIS: Item development. Pediatric Physical Therapy : The Official Publication of the Section on Pediatrics of the American Physical Therapy Association, 26(4), 385–392.
DeWitt, E. M., Stucky, B. D., Thissen, D., et al. (2011). Construction of the eight-item patient-reported outcomes measurement information system pediatric physical function scales: Built using item response theory. Journal of Clinical Epidemiology, 64(7), 794–804.
DeWalt, D. A., Gross, H. E., Gipson, D. S., et al. (2015). PROMIS((R)) pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Quality of Life Research. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 24(9), 2195–2208.
Thissen, D., Liu, Y., Magnus, B., et al. (2016). Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Quality of Life Research. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 25(1), 13–23.
Varni, J. W., Magnus, B., Stucky, B. D., et al. (2014). Psychometric properties of the PROMIS (R) pediatric scales: Precision, stability, and comparison of different scoring and administration options. Quality of Life Research. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 23(4), 1233–1243.
Howell CR, Gross HE, Reeve BB, DeWalt DA, & Huang IC (2016). known-groups validity of the patient-reported outcomes measurement information system (PROMIS) in adolescents and young adults with special healthcare needs. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 25(7), 1815–1823.
Gipson, D. S., Selewski, D. T., Massengill, S. F., et al. (2013). Gaining the PROMIS perspective from children with nephrotic syndrome: A midwest pediatric nephrology consortium study. Health and quality of life outcomes, 11, 30.
Hinds, P. S., Nuss, S. L., Ruccione, K. S., et al. (2013). PROMIS pediatric measures in pediatric oncology: Valid and clinically feasible indicators of patient-reported outcomes. Pediatric Blood & Cancer, 60(3), 402–408.
Liu, Y., Wang, J., Hinds, P. S., et al. (2015). The emotional distress of children with cancer in China: An item response analysis of C-Ped-PROMIS anxiety and depression short forms. Quality of Life Research. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 24(6), 1491–1501.
Selewski, D. T., Collier, D. N., MacHardy, J., et al. (2013). Promising insights into the health related quality of life for children with severe obesity. Health and quality of life outcomes, 11, 29.
Kratz, A. L., Slavin, M. D., Mulcahey, M. J., Jette, A. M., Tulsky, D. S., & Haley, S. M. (2013). An examination of the PROMIS((R)) pediatric instruments to assess mobility in children with cerebral palsy. Quality of Life Research, 22(10), 2865–2876.
Mulcahey MJ, Haley SM, Slavin MD, et al. (2015). Ability of PROMIS pediatric measures to detect change in children with cerebral palsy undergoing musculoskeletal surgery. Journal of Pediatric Orthopedics. 36(7), 749–756.
Centers for Disease Control and Prevention (2016). Injury prevention and control: Traumatic brain injury http://www.cdc.gov/TraumaticBrainInjury/index.html. Accessed Feb, 2016.
Cella D, Nowinski C, Peterman A, et al. The neurology quality-of-life measurement initiative. Archives of Physical Medicine and Rehabilitation. 2011;92(10, Supplement):S28–S36.
Gershon, R. C., Lai, J. S., Bode, R., et al. (2012). Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Quality of Life Research, 21(3), 475–486.
Lai, J. S., Nowinski, C., Victorson, D., et al. (2012). Quality-of-life measures in children with neurological conditions: Pediatric Neuro-QOL. Neurorehabilitation and Neural Repair, 26(1), 36–47.
Cella D (2010) Investigators N-Q. Neuro-QOL Technical Report.
Lai, J. S., Nowinski, C. J., Zelko, F., et al. (2015). Validation of the Neuro-QoL measurement system in children with epilepsy. Epilepsy & Behavior: E&B, 46, 209–214.
Tulsky, D. S., Kisala, P. A., Victorson, D., et al. (2016). TBI-QOL: Development and calibration of item banks to measure patient reported outcomes following traumatic brain injury. Journal of Head Trauma Rehabilitation, 31(1), 40–51.
Zonfrillo, M. R., Durbin, D. R., Koepsell, T. D., et al. (2014). Prevalence of and risk factors for poor functioning after isolated mild traumatic brain injury in children. Journal of Neurotrauma, 31(8), 722–727.
Green, L., Godfrey, C., Soo, C., Anderson, V., & Catroppa, C. (2012). Agreement between parent-adolescent ratings on psychosocial outcome and quality-of-life following childhood traumatic brain injury. Developmental Neurorehabilitation, 15(2), 105–113.
Varni, J. W., Seid, M., & Rode, C. A. (1999). The PedsQL: Measurement model for the pediatric quality of life inventory. Medical Care, 37(2), 126–139.
Varni, J. W., Seid, M., & Kurtin P. S. (2001). PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care, 39(8), 800–812.
Varni, J. W., Seid, M., Knight T. S., Uzark K., Szer I. S. (2002).The PedsQL 4.0 Generic core scales: Sensitivity, responsiveness, and impact on clinical decision-making. Journal of Behavioral Medicine, 25(2), 175–193.
Varni, J. W., Sherman, S. A., Burwinkle, T. M., Dickinson, P. E., & Dixon, P. (2004). The PedsQL family impact module: Preliminary reliability and validity. Health and Quality of Life Outcomes, 2, 55.
Mano, K. E. J., Khan, K. A., Ladwig, R. J., & Weisman, S. J. (2011). The impact of pediatric chronic pain on parents’ health-related quality of life and family functioning: Reliability and validity of the PedsQL 4.0 family impact module. Journal of Pediatric Psychology, 36(5), 517–527.
McCarthy, M. L., MacKenzie, E. J., Durbin, D. R., et al. (2005). The pediatric quality of life inventory: An evaluation of its reliability and validity for children with traumatic brain injury. Archives of Physical Medicine and. Rehabilitation, 86(10), 1901–1909.
Swanson, J. O., Vavilala, M. S., Wang, J., et al. (2012). Association of initial CT findings with quality-of-life outcomes for traumatic brain injury in children. Pediatric Radiology, 42(8), 974–981.
de Kloet, A. J., Lambregts, S. A., Berger, M. A., van Markus, F., Wolterbeek, R., & Vliet Vlieland, T. P. (2015). Family impact of acquired brain injury in children and youth. Journal of Developmental and Behavioral Pediatrics: JDBP, 36(5), 342–351.
Limond J, Dorris L, & McMillan TM.(2009) Quality of life in children with acquired brain injury: Parent perspectives 1–5 years after injury. Brain Injury : [BI]. 23(7):617–622.
Ayr, L. K., Yeates, K. O., Taylor, H. G., & Browne, M. (2009). Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. Journal of the International Neuropsychological Society, 15(1), 19–30.
Goodman, R. (2001). Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry, 40(11), 1337–1345.
Brooks B. L., Kadoura B., Turley B., Crawford S., Mikrogianakis A., & Barlow K. M. (2014) Perception of recovery after pediatric mild traumatic brain injury is influenced by the “good old days” bias: Tangible implications for clinical practice and outcomes research. Archives of Clinical Neuropsychology : The Official Journal of the National Academy of Neuropsychologists. 29(2), 186–193.
Iverson, G. L., Lange, R. T., Brooks, B. L., & Rennison, V. L. (2010). “Good old days” bias following mild traumatic brain injury. The Clinical Neuropsychologist, 24(1), 17–37.
Thissen, D., Varni, J. W., Stucky, B. D., Liu, Y., Irwin, D. E., & Dewalt, D. A. (2011). Using the PedsQL 3.0 asthma module to obtain scores comparable with those of the PROMIS pediatric asthma impact scale (PAIS). Quality of Life Research. An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation, 20(9), 1497–1505.
Centers for Disease Control and Prevention (1999). TBI in the United States: A report to congress. Atlanta: CDC.
Marr AL, & Coronado VG. (2004) Central Nervous System Injury Surveillance Data Submission Standards—2002. Atlanta: CDC, NCIPC;.
Faul M, & Xu L (2010) Wald MM, Coronado VG. Traumatic Brain Injury in the United States, Emergency Department Visits, Hospitalizations and Deaths, 2002–2006. Atlanta: CDC.
Carroll L. J., Cassidy J. D., Holm L., Kraus J., Coronado V. G. & Injury WHOCCTFoMTB (2004). Methodological issues and research recommendations for mild traumatic brain injury: The WHO collaborating centre task force on mild traumatic brain injury. Journal of Rehabilitation Medicine (43 Suppl):113–125.
Fay, T. B., Yeates, K. O., Taylor, H. G., et al. (2010). Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. Journal of the International Neuropsychological Society, 16(1), 94–105.
Lange, R. T., Iverson, G. L., & Franzen, M. D. (2009). Neuropsychological functioning following complicated vs. uncomplicated mild traumatic brain injury. Brain Injury, 23(2), 83–91.
Centers for Disease Control and Prevention (2003) National Center for Injury Prevention and Control. Report to congress on mild traumatic brain injury in the US: Steps to prevent a serious public health problem. Atlanta.
Gershon, R., Rothrock, N. E., Hanrahan, R. T., Jansky, L. J., Harniss, M., & Riley, W. (2010). The development of a clinical outcomes survey research application: Assessment Center. Quality of Life Research, 19(5), 677–685.
Gershon, R. C. (2005). Computer adaptive testing. Journal of Applied Measurement, 6(1), 109–127.
Organization PH. PROMIS Domain Framework. http://www.nihpromis.org/measures/domainframework2 Accessed 2/24/2016.
(NINDS) NIoNDaS (2015) Manual for the Quality of Life in Neurological Disorders (Neuro-QOL) Measures, Version 2.0.
Goodman, R., Ford, T., Simmons, H., Gatward, R., & Meltzer, H. (2000). Using the strengths and difficulties questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. The British Journal of Psychiatry : The Journal of Mental Science, 177, 534–539.
Carlsson, M., Olsson, I., Hagberg, G., & Beckung, E. (2008). Behaviour in children with cerebral palsy with and without epilepsy. Developmental Medicine & Child Neurology, 50(10), 784–789.
Clover, A. (2006). SPARCLE–a multi-centre European study of the relationship of environment to participation and quality of life in children with cerebral palsy. MBMC Public health, 6, 105.
Johnson, H., Wiggs, L., Stores, G., & Huson, S. M. (2005). Psychological disturbance and sleep disorders in children with neurofibromatosis type 1. Developmental Medicine &. Child Neurology, 47(4), 237–242.
Olsson, G. M., Marild, S., Alm, J., Brodin, U., Rydelius, P. A., & Marcus, C. (2008). The adolescent adjustment profile (AAP) in comparisons of patients with obesity, phenylketonuria or neurobehavioural disorders. Nordic Journal of Psychiatry, 62(1), 66–76.
Goodman, R. (1997). The strengths and difficulties questionnaire: A research note. Journal of Child Psychology and Psychiatry, 38(5), 581–586.
van de Looij-Jansen, P. M., Goedhart, A. W., de Wilde, E. J., & Treffers, P. D. (2010). Confirmatory factor analysis and factorial invariance analysis of the adolescent self-report strengths and difficulties questionnaire: How important are method effects and minor factors? British Journal of Clinical Psychology, 50(2), 127–144.
Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155–159.
Campbell DTF, D. (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin, 56, 81–105.
Weaver, B., & Wuensch, K. L. (2013). SPSS and SAS programs for comparing Pearson correlations and OLS regression coefficients. Behavior Research Methods, 45(3), 880–895.
Stroke NIoNDa (2014). NINDS Common Data elements: Project overview. http://www.commondataelements.ninds.nih.gov/doc/projectreview/NINDS_CDE_Overview.pdf. Accessed 26 Oct 2014.
Hunt, K. J., Alexander, I., Baumhauer, J., Brodsky, J., Chiodo, C., Daniels, T., et al. (2014) The orthopaedic foot and ankle outcomes research (OFAR) network: Feasibility of a multicenter network for patient outcomes assessment in foot and ankle. Foot & Ankle International, 35, 847–854.
Ho, B., Houck, J. R., Flemister, A. S., Ketz, J., Oh, I., DiGiovanni, B. F. et al.(2016). Preoperative PROMIS Scores predict postoperative success in foot and ankle patients. Foot & Ankle International, 37, 911–918.
Hung, M., Baumhauer, J. F., Brodsky, J. W., Cheng, C., Ellis, S. J., Franklin, J. D., et al. (2014). Psychometric comparison of the promis physical function CAT With the FAAM and FFI for measuring patient-reported outcomes. Foot & ankle international, 35, 592–599.
Hung, M., Baumhauer, J, F., Latt, L, D., Saltzman, C, L., SooHoo, N, F., Hunt, K. J. et al. (2013). Validation of PROMIS ® physical function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clinical Orthopaedics & Related Research. 471, 3466–3474.
Papuga, M. O., Mesfin, A., Molinari, R., & Rubery, P. T. (2016). Correlation of PROMIS physical function and pain cat instruments with Oswestry disability index and neck disability index in spine patients. Spine, 15, 1153–1159.
Wagner, L. I., Schink, J., Bass, M., et al. (2015). Bringing PROMIS to practice: Brief and precise symptom screening in ambulatory cancer care. Cancer, 121(6), 927–934.
Acknowledgements
This project was funded by the United States Centers for Disease Control and Prevention, Grant Number U01CE002196. The authors would like to thank Marni Levy and Natalie Jenkins for their dedicated efforts in acquiring the data described in this manuscript.
Author contributions
All the authors contributed significantly to the design, analysis, and writing of this manuscript. The contents represent the original work and have not been published elsewhere.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by the Centers for Disease Control and Prevention (Grant Number U01CE002196). The authors have no financial disclosures related to this project. Dr. David Tulsky serves on the board of the PROMIS Health Organization but does not receive compensation or royalties related to this role. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Ethical approval
Institutional Review Board (IRB) approval was attained at each of the local recruitment sites [University of Washington (Harborview Medical Center/Seattle Children’s Hospital), Nationwide Children’s Hospital, The Children’s Hospital of Philadelphia, University of Pittsburgh Medical Center’s Children’s Hospital of Pittsburgh, Children’s Healthcare of Atlanta, and Texas Children’s Hospital] and at NYU School of Medicine prior to study initiation. Parental consent and child assent (for those who were younger than age 18 at recruitment) were attained from all individual participants included at each local recruitment site prior to enrollment. Children who turned 18 during the course of this study provided informed consent as adults prior to further participation in study procedures.
Research involving human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent/assent was obtained from all individuals included in the study.
Rights and permissions
About this article
Cite this article
Bertisch, H., Rivara, F.P., Kisala, P.A. et al. Psychometric evaluation of the pediatric and parent-proxy Patient-Reported Outcomes Measurement Information System and the Neurology and Traumatic Brain Injury Quality of Life measurement item banks in pediatric traumatic brain injury. Qual Life Res 26, 1887–1899 (2017). https://doi.org/10.1007/s11136-017-1524-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-017-1524-6