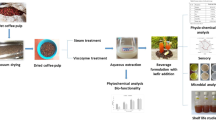
Abstract
The industrial processing of pineapples generates a substantial quantity of by-products, including shell, crown, and core. Bromelain, a proteolytic enzyme found naturally in pineapple, including its by-products, may positively influence the bioaccessibility of phenolics from milk coffee. Therefore, this study aimed to assess how the inclusion of extracts from pineapple by-products, namely shell, crown and core, could impact the bioaccessibility of coffee phenolics when combined with milk. After measuring the proteolytic activity of pineapple by-products, the standardized in vitro digestion model of INFOGEST was employed to evaluate changes in total phenolic content, total antioxidant capacity, and individual phenolic compounds in different coffee formulations. The results showed that incorporating extracts from the crown or core in both black and milk coffee increased the bioaccessibility of total phenolics (from 93 to 114% to 105–129%) and antioxidants (from 54 to 56% to 84–87%), while this effect was not observed for the shell. Moreover, adding core extracts also enhanced the bioaccessibility of caffeoylquinic acids and gallic acid in milk coffee (from 0.72 to 0.85% and 109–155%, respectively). Overall, the findings of this study highlight that bromelain from pineapple core may have a favorable effect on the recovery of phenolic compounds in milk coffee, possibly due to its ability to cleave proteins. These outcomes point out that industrial by-products can be transformed into economic value by being reintroduced into the production process through suitable treatment instead of disposal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pineapple (Ananas comosus), belonging to the Bromeliaceae family, is globally the third most consumed fruit following banana and citrus fruits [1]. Approximately 29 million tons of pineapples are produced annually, with major contributors being Indonesia, Philippines, Costa Rica, Brazil, and China [2]. Fresh pineapples have limited consumption, as 97% of the global pineapple production is utilized by the pineapple processing industry. In particular, the canning industry plays a crucial role in managing the extensive pineapple production on a global scale [3]. The industrial processing of pineapples generates a substantial quantity of by-products including shell, crown, and core [4]. By-products resulting from fruit processing compose over 50% of the total weight of the pineapple and are distributed as follows: 29–40% in the shell, 9–10% in the core, and 2–4% in the crown. While these by-products are primarily utilized in animal feed and the pharmaceutical industry, they have the potential to be transformed into value-added products. Bromelain, a proteolytic enzyme found naturally in various parts of pineapples including the shell, crown and core offers beneficial effects for both digestive and cardiovascular health [5].
Coffee (Coffea arabica L.), globally one of the most popular beverages, may be consumed either plain or with addition of milk. However, there is a debate on the effect of milk addition to coffee concerning the functional properties of the phenolic compounds. Majority of the studies have reported inhibitory effects of milk on phenolic compounds due to the potential interactions between milk proteins and coffee phenolics [6, 7]. Considering the fact that bromelain contains protease inhibitors [8], we hypothesized that incorporating extracts containing bromelain-polyphenol complex from pineapple by-products into milk coffee might have a favorable effect on recovery of phenolics besides contributing to waste valorization. In addition, the growing trend indicates a rising consumer interest in coffees with fruit-infused flavors.
Recent research not only measures the concentration of phenolic compounds along with their in vitro antioxidant capacity but also evaluates the bioaccessibility of these compounds following in vitro digestion [9,10,11]. Despite their simplicity, in vitro digestion models have proven to be valuable for predicting the outcomes of in vivo digestion [12]. However, the inconsistency in the parameters of various in vitro digestion models have restricted the comparability of findings across studies. To address this issue, INFOGEST developed a standardized static model to simulate digestion in the upper gastrointestinal tract [13, 14]. Although the INFOGEST in vitro digestion model has previously been employed to investigate how digestion of different food items affects the bioaccessibility of phenolic compounds [15], to the best of our knowledge, no previous study has investigated the effect of the addition of extracts of pineapple by-products on the bioaccessibility of coffee phenolics using the in vitro digestion model of INFOGEST.
Previous studies have focused on extraction and characterization of bromelain from pineapple by-products [16, 17], leaving gaps in specific applications of isolated enzymes in food products. Our research aimed to address this gap through incorporation of bromelain in milk coffee. In light of the above, the objective of this study was to assess the extent to which the inclusion of extracts of pineapple by-products, including shell, crown and core, could impact the bioaccessibility of coffee phenolics when combined with milk. Following the measurement of the proteolytic activity of pineapple by-products, the standardized in vitro digestion model of INFOGEST was employed to assess changes in total phenolic content, total antioxidant capacity, and individual phenolic compounds of different coffee formulations.
Materials and Methods
The materials and methods are presented in the Supplemental Material.
Results and Discussion
Proteolytic Activity
The proteolytic activities of pineapple shell, crown and core were found to be 439.9 ± 30.9, 406.3 ± 25.6, and 424.4 ± 29.1 U/mL, respectively, which were statistically not significant from each other (p > 0.05). The results obtained are observed to be higher than the data reported in the literature (85.9–199.8 U/mL) [18,19,20]. These variations could be attributed to the origin or variety of pineapple, as well as the extraction method employed. In this study, bromelain extraction was assisted with ultrasound, aiming to enhance surface contact between the pineapple by-products and solvent. Ultrasound-assisted extraction may enhance cell wall permeability through induction of oscillation, cavitation, microstreaming, and acoustic streaming, thus promoting effective mass transfer [21, 22].
Total Phenolic Content (TPC)
The changes in the TPC of coffee formulations during in vitro digestion are presented in Table 1. For undigested samples, addition of milk alone (F1) or extracts isolated from different pineapple by-products alone (F5–F7) did not cause a significant difference in the TPC of coffee (F0) (p > 0.05). Likewise, the TPC of coffee formulations containing both milk and by-product extracts (F2–F4) were not statistically different from those of the coffee alone (F0) (p > 0.05). However, the TPC of F2, containing both milk and pineapple shell extract, was found to be significantly lower than that of F5 (11%), which contained pineapple shell extract alone (p < 0.05), suggesting the inhibitory effect of milk on the recovery of phenolics in the presence of pineapple shell extract. After gastric digestion, the outcomes varied, with some formulations showing enhancement, some showing reduction, and others demonstrating no change in TPC compared to undigested formulations. After subsequent intestinal digestion, most samples exhibited higher TPC compared to those observed during gastric digestion and the bioaccessibility values varied between 91 and 129%. The increased TPC following intestinal digestion may be attributed to prolonged extraction time and/or the influence of intestinal digestive enzymes, which promote the release of phenolics [23]. The breakdown of phenolic compounds with a high degree of polymerization or conjugation in their respective derivatives could also contribute to obtained results [24]. It has been observed that the inclusion of extracts from crown or core enhances the bioaccessbility of phenolics both in black and milk coffee (from 93% (F0) to 105–116% (F6, F7) and 114% (F1) to 126–129% (F3, F4), respectively), while this effect was not observed for the shell (F2, F5). The optimal pH for bromelain isolated from pineapple stems is reported to be approximately 7, with its ideal conditions for activity ranging between 50 and 60 °C. In contrast, bromelain derived from the fruity parts exhibits an optimal pH range of 3–8, while its maximum temperature range varies widely from 37 to 70 °C [8]. As the proteolytic activity of extracts from pineapple by-products was measured only at physiological pH, it is possible that the enzyme derived from the shell may not exhibit the intended activity under simulated in vitro digestion conditions. Nevertheless, the TPC assay utilizing the Folin–Ciocalteu reagent lacks specificity for phenolic compounds. The presence of reducing agents such as ascorbic acid, citric acid, simple sugars, or specific amino acids may introduce interference with the analysis, potentially resulting in an overestimation of TPC [25]. Given this limitation, we decided to complement these findings by determining individual phenolic compounds using HPLC-PDA.
Total Antioxidant Capacity (TAC)
The changes in the TAC of coffee formulations during in vitro digestion are measured using CUPRAC and DPPH assays, and the results are presented in Table 1. Among the undigested samples, the coffee formulation containing core extract (F7) was found to possess the highest antioxidant capacity in both assays. For the CUPRAC assay results, no statistically significant differences were observed in the TAC among all other formulations (F0–F6) (p > 0.05). However, DPPH assay results showed that addition of pineapple by-product extracts to both the black and milk coffee results in reduced TAC (3–18%) in undigested samples except for F7. Following gastric digestion, the outcomes varied; however, for the majority of the samples, there was a reduction in TAC compared to undigested samples. Subsequent intestinal digestion, on the other hand, resulted in an enhanced TAC. The increase in antioxidant potential observed during the intestinal phase may be attributed to the generation of new oxidation products, exhibiting greater antioxidant activity than their precursors. Additionally, it could also result from the presence of other antioxidant molecules that are not phenolic compounds [15]. The bioaccessibility values varied between 53 and 87% and 78–112% according to DPPH and CUPRAC assays, respectively. In line with the TPC results, the inclusion of extracts from both the crown and core in black and milk coffee led to increased bioaccessibility of antioxidants, as indicated by the DPPH assay (from 54% (F0) to 84–86% (F6, F7) and 56% (F1) to 87% (F3, F4), respectively). Conversely, the CUPRAC assay results generally indicated that the addition of pineapple by-product extracts to black or milk coffee had a negative effect on the bioaccessibility of antioxidants. Both the DPPH and CUPRAC assays offer the advantages of simplicity and cost-effectiveness, requiring no specialized equipment. On the other hand, DPPH assay is only suitable for lipophilic antioxidants, whereas CUPRAC assay can measure both hydrophilic and lipophilic antioxidants. Some additional advantages of the CUPRAC assay include the fact that it is conducted at a pH level close to physiological pH. Furthermore, factors such as light, air, humidity, and pH within reasonable limits do not significantly affect the reaction. Additionally, it is more reproducible compared to other ET-based assays [25]. Nevertheless, relying on a single assay to determine the TAC of food products is not entirely reliable. Thus, conducting multiple TAC assays with different mechanisms is essential to ensure more accurate and dependable results, as demonstrated in this study using the ET-based CUPRAC and mixed-mode DPPH assays.
Individual Phenolic Compounds
In all coffee formulations, four major phenolic compounds were detected: chlorogenic acid (5-O-caffeoylquinic acid or 5-CQA), neochlorogenic acid (3-CQA), cryptochlorogenic acid (4-CQA) (Table 2), and gallic acid (Table 3). Consistent with previous reports [26, 27], chlorogenic acid was identified as the predominant compound in all coffee formulations, representing 75–77% of total CQAs. In addition, shell and crown extracts were found to contain gallic and ferulic acids (Table 3), which is also in agreement with previous findings reported in the literature [28, 29]. For undigested samples, the addition of milk alone (F1) or extracts isolated from different pineapple by-products alone (F5–F7), or their combinations (F2–F4), generally did not significantly alter the total caffeoylquinic acid and gallic acid contents compared to black coffee (F0) (p > 0.05). Gastric digestion led to significant reductions in the total caffeoylquinic acid content (p < 0.05), which further decreased during subsequent intestinal digestion. In fact, neochlorogenic and cryptochlorogenic acids were not detected after intestinal digestion. These substantial losses observed may result from the instability of CQAs in aqueous solutions. Additionally, in vitro digestion could induce the isomerization of CQAs and/or the formation of additional degradation products [30]. However, a different trend was observed for gallic acid during in vitro digestion. While gallic acid levels reduced following gastric digestion (p < 0.05), increases were noted after intestinal digestion. This observation may be related to the release of gallic acid from pineapple by-product extracts during intestinal digestion. When examining the bioaccessibility values, it becomes apparent that they are notably low for CQAs (< 1%), while for gallic acid they ranged between 52 and 155%. The incorporation of core extracts enhanced the bioaccessibility of CQAs and gallic acid in milk coffee (from 0.72% (F1) to 0.85% (F4) and 109% (F1) to 155% (F4), respectively), confirming our hypothesis regarding the favorable effect of bromelain on the recovery of phenolic compounds. On the other hand, this effect was not evident in coffee formulations containing extracts from the shell and crown. It is possible that shell and crown extracts also contain fiber, which could potentially hinder the bioaccessibility of phenolic compounds. Fiber-bound compounds tend to be poorly extracted and have limited solubility in gastrointestinal fluids [31].
Conclusions
To the best of our knowledge, no previous study has investigated the effect of the addition of pineapple by-products on the bioaccessibility of coffee phenolics using the in vitro digestion model of INFOGEST. The results revealed that incorporating extracts from the crown or core in both black and milk coffee increases the bioaccessibility of total phenolics and antioxidants. Additionally, adding core extracts also enhances the bioaccessibility of CQAs and gallic acid in milk coffee. Overall, we demonstrated that by-products of pineapple, particularly the crown and core, which are discarded as waste, have a potential to be utilized in milk coffee. However, the findings of this study are limited to the specific formulations examined. Other factors such as the proportions of coffee, milk, and pineapple by-products, as well as the temperature during mixing, could influence the bioaccessibility of phenolic compounds and need further investigation in future research. Moreover, future investigations should also conduct research on toxicological data before these materials can be utilized as food ingredients. Finally, although often ignored, the consumer acceptance of food products incorporating waste materials also should be examined.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplemental Material. Should any raw data files be needed they are available from the corresponding author upon reasonable request.
References
Abraham RA, Joshi TJ, Abdullah S (2023) A comprehensive review of pineapple processing and its by-product valorization in India. Food Chem Adv 3:100416. https://doi.org/10.1016/j.focha.2023.100416
FAOSTAT (2024) Food and Agriculture Organization Corporate Statistical Database. Crops. Retrieved from: http://www.fao.org/faostat/en/#data/QC/visualize
Dhar P, Nickhil C, Pandiselvam R, Deka SC (2023) Pineapple waste-based-biorefinery for sustainable generation of value-added products. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-04801-w
Polanía AM, Londoño L, Ramírez C et al (2023) Valorization of pineapple waste as novel source of nutraceuticals and biofunctional compounds. Biomass Convers Biorefin 13:3593–3618. https://doi.org/10.1007/s13399-022-02811-8
Santos DI, Martins CF, Amaral RA et al (2021) Pineapple (Ananas comosus L.) by-products valorization: novel bio ingredients for functional foods. Molecules 26:3216. https://doi.org/10.3390/molecules26113216
Rashidinejad A, Tarhan O, Rezaei A et al (2022) Addition of milk to coffee beverages; the effect on functional, nutritional, and sensorial properties. Crit Rev Food Sci Nutr 62:6132–6152. https://doi.org/10.1080/10408398.2021.1897516
Kamiloglu S, Ozdal T, Bakir S, Capanoglu E (2022) Bioaccessibility of terebinth (Pistacia terebinthus L.) coffee polyphenols: influence of milk, sugar and sweetener addition. Food Chem 374:131728. https://doi.org/10.1016/j.foodchem.2021.131728
Varilla C, Marcone M, Paiva L, Baptista J (2021) Bromelain, a group of pineapple proteolytic complex enzymes (Ananas comosus) and their possible therapeutic and clinical effects. A summary. Foods 10:2249. https://doi.org/10.3390/foods10102249
Dantas AM, Fernandes FG, Magnani M, da Silva Campelo Borges G (2023) Gastrointestinal digestion assays for evaluating the bioaccessibility of phenolic compounds in fruits and their derivates: an overview. Food Res Int 170:112920. https://doi.org/10.1016/j.foodres.2023.112920
Etgeton SAP, Ávila S, Silva ACR et al (2023) Nutritional composition, simulated digestion and Biological activities of Campomanesia Xanthocarpa Fruit. https://doi.org/10.1007/s11130-023-01126-x. Plant Foods Hum Nutr
Santos Pereira E dos, de Oliveira Raphaelli C, Massaut KB et al (2024) Probiotic Yogurt Supplemented with Lactococcus lactis R7 and Red Guava Extract: Bioaccessibility of Phenolic Compounds and Influence in Antioxidant Activity and Action of Alpha-amylase and Alpha-glucosidase Enzymes. Plant Foods Hum Nutr. https://doi.org/10.1007/s11130-024-01149-y
Duijsens D, Pälchen K, Guevara-Zambrano JM et al (2022) Strategic choices for in vitro food digestion methodologies enabling food digestion design. Trends Food Sci Technol 126:61–72. https://doi.org/10.1016/j.tifs.2022.06.017
Minekus M, Alminger M, Alvito P et al (2014) A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct 5:1113–1124. https://doi.org/10.1039/C3FO60702J
Brodkorb A, Egger L, Alminger M et al (2019) INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc 14. https://doi.org/10.1038/s41596-018-0119-1
Rasera GB, de Camargo AC, de Castro RJS (2023) Bioaccessibility of phenolic compounds using the standardized INFOGEST protocol: a narrative review. Compr Rev Food Sci Food Saf 22:260–286. https://doi.org/10.1111/1541-4337.13065
Aili Hamzah AF, Hamzah MH, Che Man H et al (2021) Recent updates on the Conversion of Pineapple Waste (Ananas comosus) to value-added products, Future perspectives and challenges. Agronomy 11:2221. https://doi.org/10.3390/agronomy11112221
Nath PC, Ojha A, Debnath S et al (2023) Recent advances in valorization of pineapple (Ananas comosus) processing waste and by-products: a step towards circular bioeconomy. Trends Food Sci Technol 136:100–111. https://doi.org/10.1016/j.tifs.2023.04.008
Mala T, Sadiq MB, Anal AK (2021) Comparative extraction of bromelain and bioactive peptides from pineapple byproducts by ultrasonic- and microwave‐assisted extractions. J Food Process Eng 44:e13709. https://doi.org/10.1111/jfpe.13709
Gul A, Khan S, Arain H et al (2022) Three-phase partitioning as an efficient one‐step method for the extraction and purification of bromelain from pineapple crown waste. J Food Process Preserv 46:e16973. https://doi.org/10.1111/jfpp.16973
Kaushal R, Kaur B, Panesar PS et al (2023) Valorization of pineapple rind for bromelain extraction using microwave assisted technique: optimization, purification, and structural characterization. J Food Sci Technol. https://doi.org/10.1007/s13197-023-05863-4
Yolci Omeroglu P, Acoglu B, Özdal T et al (2019) Extraction techniques for plant-based bio-active compounds. In: Swamy MK, Akhtar MS (eds) Natural bio-active compounds. Springer Singapore, Singapore, pp 465–492
Singla M, Sit N (2021) Application of ultrasound in combination with other technologies in food processing: a review. Ultrason Sonochem 73:105506. https://doi.org/10.1016/j.ultsonch.2021.105506
Kamiloglu S, Koc Alibasoglu E, Acoglu Celik B et al (2024) Bioaccessibility of carotenoids and polyphenols in Organic Butternut Squash (Cucurbita moschata): impact of industrial freezing process. Foods 13:239. https://doi.org/10.3390/foods13020239
Eran Nagar E, Berenshtein L, Hanuka Katz I et al (2021) The impact of chemical structure on polyphenol bioaccessibility, as a function of processing, cell wall material and pH: a model system. J Food Eng 289:110304. https://doi.org/10.1016/j.jfoodeng.2020.110304
Capanoglu E, Kamiloglu S, Cekic SD et al (2022) Antioxidant activity and capacity measurement. In: Ekiert HM, Ramawa KG, Arora J (eds) Plant antioxidants and Health. Springer, pp 709–773
Vilas-Boas AA, Oliveira A, Jesus D et al (2020) Chlorogenic acids composition and the impact of in vitro gastrointestinal digestion on espresso coffee from single-dose capsule. Food Res Int 134:109223. https://doi.org/10.1016/j.foodres.2020.109223
Pedan V, Stamm E, Do T et al (2020) HPTLC fingerprint profile analysis of coffee polyphenols during different roast trials. J Food Compost Anal 94:103610. https://doi.org/10.1016/j.jfca.2020.103610
Brito TBN, Lima RS, Santos LB MC, et al (2021) Antimicrobial, antioxidant, volatile and phenolic profiles of cabbage-stalk and pineapple-crown flour revealed by GC-MS and UPLC-MSE. Food Chem 339:127882. https://doi.org/10.1016/j.foodchem.2020.127882
Lourenço SC, Campos DA, Gómez-García R et al (2021) Optimization of natural antioxidants extraction from Pineapple Peel and their stabilization by spray drying. Foods 10:1255. https://doi.org/10.3390/foods10061255
Ianni F, Barola C, Blasi F et al (2022) Investigation on chlorogenic acid stability in aqueous solution after microwave treatment. Food Chem 374:131820. https://doi.org/10.1016/j.foodchem.2021.131820
Kamiloglu S, Ozdal T, Tomas M, Capanoglu E (2022) Oil matrix modulates the bioaccessibility of polyphenols: a study of salad dressing formulation with industrial broccoli by-products and lemon juice. J Sci Food Agric 102:5368–5377. https://doi.org/10.1002/jsfa.11890
Funding
No funding was received for conducting this study.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Z.B.K. did investigations; G.O. did the conceptualization, formal analysis, project administration, writing—review and editing; S. K. did the formal analysis, writing—original draft preparation; E.C. did the conceptualization, project administration, resources, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kocakaplan, Z.B., Ozkan, G., Kamiloglu, S. et al. Valorization of Pineapple (Ananas comosus) By-Products in Milk Coffee Beverage: Influence on Bioaccessibility of Phenolic Compounds. Plant Foods Hum Nutr 79, 300–307 (2024). https://doi.org/10.1007/s11130-024-01183-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-024-01183-w