Abstract
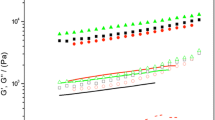
Cajanus cajan [pigeon pea (PP)] is an important legume crop for subsistence agriculture and its seeds are an alternative plant-based protein source. PP protein isolates (PPI) are able to form heat-induced gels that could be used for food applications. The aim of this work was to study the influence of pH (2.1, 3.9, 6.3, and 8.3) and ionic strength (μ) (0.10 and 0.54) on thermal stability and thermal gelation of PPI obtained by alkaline extraction (pH 8.0) and isoelectric precipitation. Thermal stability of PPI changed with pH variation at low ionic strength (μ = 0.10), decreasing this dependence with the increase of ionic strength (μ = 0.54). At μ = 0.10, gelation capacity of PPI was lower at pH 2.1 and pH 3.9. These gels presented a coarse network, which entails low WHC. At pH 6.3 and pH 8.3, gels showed a solid-like character with a compact and homogeneous matrix, with better WHC. At μ = 0.54, gel formation was favoured at pH 2.1 and pH 3.9. G′20/G′95 ratio values and differential solubility results suggest that hydrogen bonds and electrostatic interactions could play an important role in gel formation at pH 6.3 and pH 8.3.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DSC:
-
Differential scanning calorimeter
- ESM:
-
Electronical Supplementary Material
- G′:
-
Elastic modulus
- G″:
-
Viscous modulus
- LGC:
-
Least gelation concentration
- pI:
-
Isoelectric point
- PP:
-
Pigeon pea
- PPI:
-
Pigeon pea protein isolates
- SB:
-
Saline buffer
- So:
-
Protein solubility
- tan δ:
-
Tangent of the phase angle
- U:
-
Urea
- W:
-
Water
References
Sá AGA, Moreno YMF, Carciofi BAM (2020) Plant proteins as high-quality nutritional source for human diet. Trends Food Sci Technol 97:170–184. https://doi.org/10.1016/j.tifs.2020.01.011
Mula MG, Saxena KB (2010) Potential of Pigeonpea: The crop, ecology, utilization, importance in cropping systems and nutritional quality. In: Lifting the level of awareness on pigeonpea – a global perspective. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Patancheru, pp 20–66
Krishnan HB, Natarajan SS, Oehrle NW, Garrett WM, Darwish O (2017) Proteomic analysis of pigeonpea (Cajanus cajan) seeds reveals the accumulation of numerous stress-related proteins. J Agric Food Chem 65:4572–4581. https://doi.org/10.1021/acs.jafc.7b00998
Fernández Sosa EI, Chaves MG, Quiroga AV, Avanza MV (2021) Comparative study of structural and physicochemical properties of pigeon pea (Cajanus cajan L.) protein isolates and its major protein fractions. Plant Foods Hum Nutr 76:37–45. https://doi.org/10.1007/s11130-020-00871-7
Fernández Sosa EI, Chaves MG, Henao Ossa JS, Quiroga AV, Avanza MV (2021) Protein isolates from Cajanus cajan L. as surfactant for o:w emulsions: pH and ionic strength influence on protein structure and emulsion stability. Food Biosci 42:101159. https://doi.org/10.1016/j.fbio.2021.101159
Syed R, Ding HH, Hui D, Wu Y (2022) Physicochemical and functional properties of pigeon pea (Cajanus cajan) protein and non-starch polysaccharides. Bioact Carbohydr Dietary Fibre 28:100317. https://doi.org/10.1016/j.bcdf.2022.100317
Gomezulu AD, Mongi RJ (2022) Protein content and anti-nutritional factors in pigeon pea and effect of its protein isolate on physical properties and consumer preference of beef sausages. Appl Food Res 2:100047. https://doi.org/10.1016/j.afres.2022.100047
Tanger C, Müller M, Andlinger D, Kulozik U (2022) Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocoll 123:106903. https://doi.org/10.1016/j.foodhyd.2021.106903
Peyrano F, de Lamballerie M, Avanza MV, Speroni F (2022) High hydrostatic pressure- or heat-induced gelation of cowpea proteins at low protein content: effect of calcium concentration. Food Hydrocoll 124:107220. https://doi.org/10.1016/j.foodhyd.2021.107220
Dyer JM, Nelson JW, Murai N (1992) Biophysical analysis of Phaseolin denaturation induced by urea, Guanidinium chloride, pH, and temperature. J Protein Chem 11:281–288. https://doi.org/10.1007/BF01024867
Ma CY, Harwalkar VR (1991) Thermal analysis of food proteins. In advances in food and nutrition research. Academic Press 35:317–366. https://doi.org/10.1016/S1043-4526(08)60067-4
Peyrano F, de Lamballerie M, Avanza MV, Speroni F (2017) Calorimetric study of cowpea protein isolates. Effect of calcium and high hydrostatic pressure. Food Biophy 12:374–382. https://doi.org/10.1007/s11483-017-9493-4
Sun XD, Arntfield SD (2011) Dynamic oscillatory rheological measurement and thermal properties of pea protein extracted by salt method: effect of pH and NaCl. J Food Eng 105:577–582. https://doi.org/10.1016/j.jfoodeng.2011.03.008
Castellani OF, Martínez EN, Añón MC (1998) Structural modifications of an amaranth globulin induced by pH and NaCl. J Agric Food Chem 46(12):4846–4853. https://doi.org/10.1021/jf9802427
Shand PJ, Ya H, Pietrasik Z, Wanasundara PKJPD (2007) Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem 102:1119–1130. https://doi.org/10.1016/j.foodchem.2006.06.060
Zhang YH, Tang CH, Wen QB, Yang XQ, Li L, Deng WL (2010) Thermal aggregation and gelation of kidney bean (Phaseolus vulgaris L.) protein isolate at pH 2.0: influence of ionic strength. Food Hydrocoll 24:266–274. https://doi.org/10.1016/j.foodhyd.2009.10.002
Yang J, Zamani S, Liang L, Chen L (2021) Extraction methods significantly impact pea protein composition, structure and gelling properties. Food Hydrocoll 117:106678. https://doi.org/10.1016/j.foodhyd.2021.106678
Mwasaru MA, Muhammad K, Bakar J, Man YBC (1999) Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeonpea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. II Functional properties Food Chem 67(4):445–452. https://doi.org/10.1016/S0308-8146(99)00151-X
Tang X, Shen Y, Zhang Y, Schilling MW, Li Y (2021) Parallel comparison of functional and physicochemical properties of common pulse proteins. LWT - Food Sci Technol 146:111594. https://doi.org/10.1016/j.lwt.2021.111594
Peyrano F, de Lamballerie M, Speroni F, Avanza MV (2019) Rheological characterization of thermal gelation of cowpea protein isolates: effect of processing conditions. LWT - Food Sci Technol 109:406–414. https://doi.org/10.1016/j.lwt.2019.04.048
Zhang T, Jiang B, Wang Z (2007) Gelation properties of chickpea protein isolates. Food Hydrocoll 21:280–286. https://doi.org/10.1016/j.foodhyd.2006.04.005
Clark AH, Ross-Murphy SB (1987) Structural and mechanical properties of biopolymer gels. Adv Polym Sci 83:60–192. https://doi.org/10.1533/9781845698331.322
Moreno HM, Dominguez-Timon F, Díaz MT, Pedrosa MM, Borderías AJ, Tovar CA (2020) Evaluation of gels made with different commercial pea protein isolate: rheological, structural and functional properties. Food Hydrocoll 99:105375. https://doi.org/10.1016/j.foodhyd.2019.105375
Kavanagh G, Ross-Murphy SB (1998) Rheological characterization of polymer gels. Prog Polym Sci 23:533–562. https://doi.org/10.1016/S0079-6700(97)00047-6
Piñeiro-Lago L, Franco I, Tovar CA (2020) Changes in thermoviscoelastic and biochemical properties of Atroncau blancu and roxu Afuega'l Pitu cheese (PDO) during ripening. Food Res Int 137:109693. https://doi.org/10.1016/j.foodres.2020.109693
Britten M, Giroux HJ (2001) Acid-induced gelation of whey protein polymers: effects of pH and calcium concentration during polymerization. Food Hydrocoll 15:609–617. https://doi.org/10.1016/S0268-005X(01)00049-2
Klost M, Brzeski C, Drusch S (2020) Effect of protein aggregation on rheological properties of pea protein gels. Food Hydrocoll 108:106036. https://doi.org/10.1016/j.foodhyd.2020.106036
Andlinger DJ, Bornkeßel AC, Jung I, Schroeter B, Smirnova I, Kulozik U (2021) Microstructures of potato protein hydrogels and aerogels produced by thermal crosslinking and supercritical drying. Food Hydrocoll 112:106305. https://doi.org/10.1016/j.foodhyd.2020.106305
Zhao ZK, Mu TH, Zhang M, Richel A (2018) Chemical forces, structure, and gelation properties of sweet potato protein as affected by pH and high hydrostatic pressure. Food Bioprocess Technol 11:1719–1732. https://doi.org/10.1007/s11947-018-2137-y
Langton M, Ehsanzamir S, Karkehabadi S, Feng X, Johansson M, Johansson DP (2020) Gelation of faba bean proteins-effect of extraction method, pH and NaCl. Food Hydrocoll 103:105622. https://doi.org/10.1016/j.foodhyd.2019.105622
Puppo MC, Añón MC (1998) Structural properties of heat-induced soy protein gels as affected by ionic strength and pH. J Agric Food Chem 46:3583–3589. https://doi.org/10.1021/jf980006w
Potin F, Lubbers S, Husson F, Saurel R (2019) Hemp (Cannabis sativa L.) protein extraction conditions affect extraction yield and protein quality. J Food Sci 84:3682–3690. https://doi.org/10.1111/1750-3841.14850
Bryant CM, McClements DJ (1998) Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci Technol 9:143–151. https://doi.org/10.1016/S0924-2244(98)00031-4
Renkema JM, Lakemond CM, De Jongh HH, Gruppen H, van Vliet T (2000) The effect of pH on heat denaturation and gel forming properties of soy proteins. J Biotechnol 79:223–230. https://doi.org/10.1016/S0168-1656(00)00239-X
Ge J, Sun C, Chang Y, Li S, Zhang Y, Fang Y (2023) Understanding the differences in heat-induced gel properties of twelve legume proteins: a comparative study. Food Res Int 163:112134. https://doi.org/10.1016/j.foodres.2022.112134
Acknowledgments
The authors wish to thank Juan Manuel Castagnini and Mercedes Carolina Rasia from the Facultad de Ciencias de la Alimentación (UNER) for their kind help and suggestions during experiments.
Funding
The authors acknowledge the financial support from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and the Universidad Nacional del Nordeste (UNNE) and CONICET, Argentina.
Author information
Authors and Affiliations
Contributions
Fernández Sosa EI. Methodology, Validation, Formal analysis, Investigation, Data curation, Writing - Original Draft; Chaves MG. Conceptualization, Methodology, Writing - Review & Editing, Visualization, Supervision, Funding acquisition; Peyrano F. Methodology, Validation, Writing - Review & Editing, Visualization; Quiroga AV. Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing, Visualization; Avanza MV. Conceptualization, Resources, Writing - Review & Editing Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors agreed to publish the results in Plant Foods for Human Nutrition.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández Sosa, E.I., Chaves, M.G., Peyrano, F. et al. Thermal Gelation of Proteins from Cajanus cajan Influenced by pH and Ionic Strength. Plant Foods Hum Nutr 78, 574–583 (2023). https://doi.org/10.1007/s11130-023-01086-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01086-2