Abstract
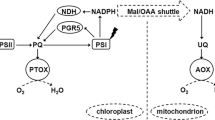
Cotton (Gossypium hirsutum L.) leafroll dwarf virus disease (CLRDD) is a yield-limiting threat to cotton production and can substantially limit net photosynthetic rates (AN). Previous research showed that AN was more sensitive to CLRDD-induced reductions in stomatal conductance than electron transport rate (ETR) through photosystem II (PSII). This observation coupled with leaf reddening symptomology led to the hypothesis that differential sensitivities of photosynthetic component processes to CLRDD would contribute to declines in AN and increases in oxidative stress, stimulating anthocyanin production. Thus, an experiment was conducted to define the relative sensitivity of photosynthetic component processes to CLRDD and to quantify oxidative stress and anthocyanin production in field-grown cotton. Among diffusional limitations to AN, reductions in mesophyll conductance and CO2 concentration in the chloroplast were the greatest constraints to AN under CLRDD. Multiple metabolic processes were also adversely impacted by CLRDD. ETR, RuBP regeneration, and carboxylation were important metabolic (non-diffusional) limitations to AN in symptomatic plants. Photorespiration and dark respiration were less sensitive than photosynthetic processes, contributing to declines in AN in symptomatic plants. Among thylakoid processes, reduction of PSI end electron acceptors was the most sensitive to CLRDD. Oxidative stress indicators (H2O2 production and membrane peroxidation) and anthocyanin contents were substantially higher in symptomatic plants, concomitant with reductions in carotenoid content and no change in energy dissipation by PSII. We conclude that differential sensitivities of photosynthetic processes to CLRDD and limited potential for energy dissipation at PSII increases oxidative stress, stimulating anthocyanin production as an antioxidative mechanism.







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author (Ved Parkash), upon request.
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ 24(12):1337–1344
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Avelar S, Schrimsher D, Lawrence K, Brown JK (2019) First report of Cotton leafroll dwarf virus associated with cotton blue disease symptoms in Alabama. Plant Dis 103(3):592–592
Avelar S, Ramos-Sobrinho R, Conner K, Nichols RL, Lawrence K, Brown JK (2020) Characterization of the complete genome and p0 protein for a previously unreported genotype of cotton leafroll dwarf virus, an introduced polerovirus in the United States. Plant Dis 104(3):780–786
Bag S, Roberts PM, Kemerait RC (2021) cotton leafroll dwarf disease: an emerging virus disease on cotton in the US. Crops Soils 54(2):18–22
Balachandran S, Osmond CB, Makino A (1994) Effects of two strains of tobacco mosaic virus on photosynthetic characteristics and nitrogen partitioning in leaves of Nicotiana tabacum cv Xanthi during photoacclimation under two nitrogen nutrition regimes. Plant Physiol 104(3):1043–1050
Banks JM (2018) Chlorophyll fluorescence as a tool to identify drought stress in acer genotypes. Environ Exp Bot 155:118–127
Basso MF, Fajardo TV, Santos HP, Guerra CC, Ayub RA, Nickel O (2010) Leaf physiology and enologic grape quality of virus-infected plants. Trop Plant Pathol 35(6):351–359
Brestic M, Zivcak M, Kalaji HM, Carpentier R, Allakhverdiev SI (2012) Photosystem II thermostability in situ: environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol Biochem 57:93–105
Ceppi MG, Oukarroum A, Çiçek N, Strasser RJ, Schansker G (2012) The IP amplitude of the fluorescence rise OJIP is sensitive to changes in the photosystem I content of leaves: a study on plants exposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol Plant 144(3):277–288
Chastain DR, Snider JL, Collins GD, Perry CD, Whitaker J, Byrd SA (2014) Water deficit in field-grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. J Plant Physiol 171(17):1576–1585
Chastain DR, Snider JL, Choinski JS, Collins GD, Perry CD, Whitaker J, Grey TL, Sorensen RB, van Iersel M, Byrd SA (2016) Leaf ontogeny strongly influences photosynthetic tolerance to drought and high temperature in Gossypium hirsutum. J Plant Physiol 199:18–28
Close DC, Beadle CL (2003) The ecophysiology of foliar anthocyanin. Bot Rev 69(2):149–161
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(1):93–101
Dillenburg LR, Sullivan JH, Teramura AH (1995) Leaf expansion and development of photosynthetic capacity and pigments in liquidambar styraciflua (Hamamelidaceae)—effects of UV-B radiation. Am J Bot 82(7):878–885
Duursma RA (2015) Plantecophys—an R package for analysing and modelling leaf gas exchange data. PLoS ONE 10(11):e0143346
Earl HJ, Ennahli S (2004) Estimating photosynthetic electron transport via chlorophyll fluorometry without photosystem II light saturation. Photosynth Res 82(2):177–186
Edula SR, Bag S, Milner H, Kumar M, Suassuna ND, Chee PW, Kemerait RC, Hand LC, Snider JL, Srinivasan R, Roberts PM (2023) Cotton leafroll dwarf disease: an enigmatic viral disease in cotton. Mol Plant Pathol 24(6):513–526
El Aou-ouad H, Montero R, Medrano H, Bota J (2016) Interactive effects of grapevine leafroll-associated virus 3 (GLRaV-3) and water stress on the physiology of Vitis vinifera L. cv. Malvasia de Banyalbufar and Giro-Ros. J Plant Physiol 196–197:106–115
Ennahli S, Earl HJ (2005) Physiological limitations to photosynthetic carbon assimilation in cotton under water stress. Crop Sci 45(6):2374–2382
Epron D, Godard D, Cornic G, Genty B (1995) Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Fagus sylvatica L and Castanea sativa Mill). Plant, Cell Environ 18(1):43–51
Flexas J, Clemente-Moreno MJ, Bota J, Brodribb TJ, Gago J, Mizokami Y, Nadal M, Perera-Castro AV, Roig-Oliver M, Sugiura D, Xiong D, Carriquí M (2021) Cell wall thickness and composition are involved in photosynthetic limitation. J Exp Bot 72(11):3971–3986
Galmés J, Medrano H, Flexas J (2007) Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol 175(1):81–93
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990(1):87–92
Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem Photobiol 74(1):38–45
Gould KS (2004) Nature’s Swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 2004(5):314
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Hasanuzzaman M, Nahar K, Gill SS, Fujita M (2013) Drought stress responses in plants, oxidative stress, and antioxidant defense. In: Climate change and plant abiotic stress tolerance, pp 209–250
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2005) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57(2):291–302
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Brestic M, Bussotti F, Calatayud A, Dąbrowski P, Elsheery NI, Ferroni L, Guidi L, Hogewoning SW, Jajoo A, Misra AN, Nebauer SG, Pancaldi S, Penella C, Poli D, Pollastrini M, Romanowska-Duda ZB, Rutkowska B, Serôdio J, Suresh K, Szulc W, Tambussi E, Yanniccari M, Zivcak M (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122(2):121–158
Khan MA, Wahid A, Ahmad M, Tahir MT, Ahmed M, Ahmad S, Hasanuzzaman M (2020) World cotton production and consumption: an overview. In: Ahmad S, Hasanuzzaman M (eds) Cotton production and uses: agronomy, crop protection, and postharvest technologies. Springer, Singapore, pp 1–7
Lawlor DW (2002) Limitation to photosynthesis in water-stressed leaves: stomata vs metabolism and the role of ATP. Ann Bot 89(7):871–885
Lei Z, Liu F, Wright IJ, Carriquí M, Niinemets Ü, Han J, Jia M, Atwell BJ, Cai X, Zhang W, Zhou Z, Zhang Y (2021) Comparisons of photosynthetic and anatomical traits between wild and domesticated cotton. J Exp Bot 73(3):873–885
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd
Loka DA, Oosterhuis DM (2010) Effect of high night temperatures on cotton respiration, ATP levels and carbohydrate content. Environ Exp Bot 68(3):258–263
Marquardt J, Bassi R (1993) Chlorophyll-proteins from maize seedlings grown under intermittent light conditions. Planta 191(2):265–273
Montero R, Elaououad H, Pacifico D, Marzachì C, Castillo N, García E, Del Saz NF, Florez-Sarasa I, Flexas J, Bota J (2017) Effects of Grapevine leafroll-associated virus 3 on the physiology in asymptomatic plants of Vitis vinifera. Ann Appl Biol 171(2):155–171
Moutinho-Pereira J, Correia C, Gonçalves B, Bacelar E, Coutinho J, Ferreira H, Lousada J, Cortez M (2012) Impacts of leafroll-associated viruses (GLRaV-1 and-3) on the physiology of the P ortuguese grapevine cultivar ‘T ouriga N acional’growing under field conditions. Ann Appl Biol 160(3):237–249
Naing AH, Kim CK (2021) Abiotic stress-induced anthocyanins in plants: their role in tolerance to abiotic stresses. Physiol Plant 172(3):1711–1723
Oukarroum A, Schansker G, Strasser RJ (2009) Drought stress effects on photosystem I content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol Plant 137(2):188–199
Oukarroum A, El-Madidi S, Strasser RJ (2012) Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L): a chlorophyll a fluorescence study. Plant Biosyst: Int J Deal Aspects Plant Biol 146(4):1037–1043
Parkash V, Sharma DB, Snider J, Bag S, Roberts P, Tabassum A, West D, Khanal S, Suassuna N, Chee P (2021) Effect of cotton leafroll dwarf virus on physiological processes and yield of individual cotton plants. Front Plant Sci. https://doi.org/10.3389/fpls.2021.734386
Parkash V, Snider JL, Sintim HY, Hand LC, Virk G, Pokhrel A (2023) Differential sensitivities of photosynthetic processes and carbon loss mechanisms govern N-induced variation in net carbon assimilation rate for field-grown cotton. J Exp Bot 74(8):2638–2652
Pilon C, Snider JL, Sobolev V, Chastain DR, Sorensen RB, Meeks CD, Massa AN, Walk T, Singh B, Earl HJ (2018) Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J Plant Physiol 231:124–134
Pospíšil P (2009) Production of reactive oxygen species by photosystem II. Biochim Biophys Acta: Bioenergetics 1787(10):1151–1160
Rahoutei J, García-Luque I, Barón M (2000) Inhibition of photosynthesis by viral infection: effect on PSII structure and function. Physiol Plant 110(2):286–292
Roig-Oliver M, Bresta P, Nadal M, Liakopoulos G, Nikolopoulos D, Karabourniotis G, Bota J, Flexas J (2020) Cell wall composition and thickness affect mesophyll conductance to CO2 diffusion in Helianthus annuus under water deprivation. J Exp Bot 71(22):7198–7209
Roig-Oliver M, Fullana-Pericàs M, Bota J, Flexas J (2021) Adjustments in photosynthesis and leaf water relations are related to changes in cell wall composition in Hordeum vulgare and Triticum aestivum subjected to water deficit stress. Plant Sci 311:111015
Ryšlavá H, Müller K, Semorádová Š, Synková H, Čeřovská N (2003) Photosynthesis and activity of phospho enol pyruvate carboxylase in Nicotiana tabacum L leaves infected by potato virus A and potato virus Y. Photosynthetica 41(3):357–363
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120(2):179–186
Sampol B, Bota J, Riera D, Medrano H, Flexas J (2003) Analysis of the virus-induced inhibition of photosynthesis in malmsey grapevines. New Phytol 160(2):403–412
Sharman M, Lapbanjob S, Sebunruang P, Belot JL, Galbieri R, Giband M, Suassuna N (2015) First report of cotton leafroll dwarf virus in Thailand using a species-specific PCR validated with isolates from Brazil. Aust Plant Dis Notes 10(1):24
Silva T, Corrêa R, Castilho Y, Silvie P, Bélot J-L, Vaslin M (2008) Widespread distribution and a new recombinant species of Brazilian virus associated with cotton blue disease. Virol J 5(1):1–13
Snider JL, Thangthong N, Pilon C, Virk G, Tishchenko V (2018) OJIP-fluorescence parameters as rapid indicators of cotton (Gossypium hirsutum L.) seedling vigor under contrasting growth temperature regimes. Plant Physiol Biochem 132:249–257
Snider J, Harris G, Roberts P, Meeks C, Chastain D, Bange M, Virk G (2021) Cotton physiological and agronomic response to nitrogen application rate. Field Crop Res 270:108194
Snider JL, Pilon C, Hu W, Wang H-M, Tishchenko V, Slaton W, Chastain D, Parkash V (2022) Net photosynthesis acclimates to low growth temperature in cotton seedlings by shifting temperature thresholds for photosynthetic component processes and respiration. Environ Exp Bot 196:104816
Song X-S, Wang Y-J, Mao W-H, Shi K, Zhou Y-H, Nogués S, Yu J-Q (2009) Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves. Physiol Plant 135(3):246–257
Souza PF, Silva FD, Carvalho FE, Silveira JA, Vasconcelos IM, Oliveira JT (2017) Photosynthetic and biochemical mechanisms of an EMS-mutagenized cowpea associated with its resistance to cowpea severe mosaic virus. Plant Cell Rep 36(1):219–234
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta: Bioenergetics 1797(6):1313–1326
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing photosynthesis: mechanisms, regulation and adaptation, pp. 445–483
Strauss AJ, Krüger GHJ, Strasser RJ, Heerden PDRV (2006) Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P. Environ Exp Bot 56(2):147–157
Tabassum A, Bag S, Roberts P, Suassuna N, Chee P, Whitaker J, Conner K, Brown J, Nichols R, Kemerait R (2019) First report of cotton leafroll dwarf virus infecting cotton in Georgia, USA. Plant Dis 103(7):1803
Tabassum A, Roberts PM, Bag S (2020) Genome sequence of cotton leafroll dwarf virus infecting cotton in Georgia, USA. Microbiol Resour Announcements 9(34):e00812-00820
Taulavuori E, Hellström EK, Taulavuori K, Laine K (2001) Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L) during snow removal, reacclimation and cold acclimation. J Exp Bot 52(365):2375–2380
University of Georgia (2021) Georgia cotton production guide. The University of Georgia Cooperative Extension. http://www.ugacotton.com/production-guide/
Valentini R, Epron D, De Angelis P, Matteucci G, Dreyer E (1995) In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L) leaves: diurnal cycles under different levels of water supply. Plant, Cell Environ 18(6):631–640
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151(1):59–66
Virk G, Snider JL, Chee P, Jespersen D, Pilon C, Rains G, Roberts P, Kaur N, Ermanis A, Tishchenko V (2021) Extreme temperatures affect seedling growth and photosynthetic performance of advanced cotton genotypes. Ind Crops Prod 172:114025
Woodrow IE, Berry J (1988) Enzymatic regulation of photosynthetic CO2, fixation in C3 plants. Annu Rev Plant Physiol Plant Mol Biol 39(1):533–594
Xu Z, Rothstein SJ (2018) ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav 13(3):e1451708
Xu Y, Xu Q, Huang B (2015) Ascorbic acid mitigation of water stress-inhibition of root growth in association with oxidative defense in tall fescue (Festuca arundinacea Schreb). Front Plant Sci. https://doi.org/10.3389/fpls.2015.00807
Xu Z, Mahmood K, Rothstein SJ (2017) ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol 58(8):1364–1377
Yamori W, Nagai T, Makino A (2011) The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant, Cell Environ 34(5):764–777
Zahoor R, Zhao W, Dong H, Snider JL, Abid M, Iqbal B, Zhou Z (2017) Potassium improves photosynthetic tolerance to and recovery from episodic drought stress in functional leaves of cotton (Gossypium hirsutum L.). Plant Physiol Biochem 119:21–32
Zhou YH, Peng YH, Lei JL, Zou LY, Zheng JH, Yu JQ (2004) Effects of potato virus YNTN infection on gas exchange and photosystem 2 function in leaves of Solanum tuberosum L. Photosynthetica 42(3):417–423
Zou J, Hu W, Li Y, Zhu H, He J, Wang Y, Meng Y, Chen B, Zhao W, Wang S, Zhou Z (2022) Leaf anatomical alterations reduce cotton’s mesophyll conductance under dynamic drought stress conditions. Plant J 111(2):391–405
Acknowledgements
The authors would like to thank Cotton Incorporated, Georgia Cotton Commission, and the University of Georgia for their financial and material support of this research. We would also like to thank Lola Sexton, Will Vance, Devendra Prasad Chalise, Amrit Pokhrel, Joshua Lee, and Navneet Kaur for their support in the field.
Author information
Authors and Affiliations
Contributions
Conceptualization: JS and VP; Data curation: JS and VP; Formal analysis: JS, and VP; Funding acquisition: JS; Investigation: JS, VP, GV, SB, CP, and KD; Methodology: JS and VP; Project administration: JS and VP; Resources: JS, CP, SB, and DJ; Writing—Original draft: VP and JS; Writing—review and editing: JS, VP, CP, SB, DJ, GV, and KD.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parkash, V., Snider, J.L., Pilon, C. et al. Differential sensitivities of photosynthetic component processes govern oxidative stress levels and net assimilation rates in virus-infected cotton. Photosynth Res 158, 41–56 (2023). https://doi.org/10.1007/s11120-023-01038-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-023-01038-6