Abstract
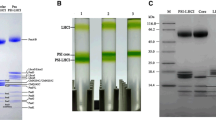
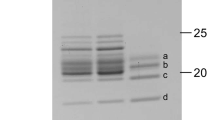
Light-harvesting complexes (LHCs) have been diversified in oxygenic photosynthetic organisms, and play an essential role in capturing light energy which is transferred to two types of photosystem cores to promote charge-separation reactions. Red algae are one of the groups of photosynthetic eukaryotes, and their chlorophyll (Chl) a-binding LHCs are specifically associated with photosystem I (PSI). In this study, we purified three types of preparations, PSI-LHCI supercomplexes, PSI cores, and isolated LHCIs, from the red alga Cyanidium caldarium, and examined their properties. The polypeptide bands of PSI-LHCI showed characteristic PSI and LHCI components without contamination by other proteins. The carotenoid composition of LHCI displayed zeaxanthins, β-cryptoxanthins, and β-carotenes. Among the carotenoids, zeaxanthins were enriched in LHCI. On the contrary, both zeaxanthins and β-cryptoxanthins could not be detected from PSI, suggesting that zeaxanthins and β-cryptoxanthins are bound to LHCI but not PSI. A Qy peak of Chl a in the absorption spectrum of LHCI was shifted to a shorter wavelength than those in PSI and PSI-LHCI. This tendency is in line with the result of fluorescence-emission spectra, in which the emission maxima of PSI-LHCI, PSI, and LHCI appeared at 727, 719, and 677 nm, respectively. Time-resolved fluorescence spectra of LHCI represented no 719 and 727-nm fluorescence bands from picoseconds to nanoseconds. These results indicate that energy levels of Chls around/within LHCIs and within PSI are changed by binding LHCIs to PSI. Based on these findings, we discuss the expression, function, and structure of red algal PSI-LHCI supercomplexes.






Similar content being viewed by others
Abbreviations
- β-DDM:
-
n-Dodecyl-β-d-maltoside
- Chl:
-
Chlorophyll
- LHC:
-
Light-harvesting complex
- LHCI:
-
LHC specific to PSI
- PS:
-
Photosystem
- TRF:
-
Time-resolved fluorescence
References
Abram M, Białek R, Szewczyk S, Karolczak J, Gibasiewicz K, Kargul J (2020) Remodeling of excitation energy transfer in extremophilic red algal PSI-LHCI complex during light adaptation. Biochim Biophys Acta, Bioenerg 1861:148093
Akimoto S, Yokono M, Ohmae M, Yamazaki I, Tanaka A, Higuchi M, Tsuchiya T, Miyashita H, Mimuro M (2005) Ultrafast excitation relaxation dynamics of lutein in solution and in the light-harvesting complexes II isolated from Arabidopsis thaliana. J Phys Chem B 109:12612–12619
Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A (2012) Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta, Bioenerg 1817:1483–1489
Akimoto S, Teshigahara A, Yokono M, Mimuro M, Nagao R, Tomo T (2014) Excitation relaxation dynamics and energy transfer in fucoxanthin-chlorophyll a/c-protein complexes, probed by time-resolved fluorescence. Biochim Biophys Acta, Bioenerg 1837:1514–1521
Allen MB (1959) Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol 32:270–277
Antoshvili M, Caspy I, Hippler M, Nelson N (2019) Structure and function of photosystem I in Cyanidioschyzon merolae. Photosynth Res 139:499–508
Blankenship RE (2021) Molecular mechanisms of photosynthesis, 3rd edn. Wiley, Blackwell, Oxford, UK
Breithaupt DE, Bamedi A (2001) Carotenoid esters in vegetables and fruits: a screening with emphasis on β-cryptoxanthin esters. J Agric Food Chem 49:2064–2070
Brettel K, Leibl W (2001) Electron transfer in photosystem I. Biochim Biophys Acta, Bioenerg 1507:100–114
Busch A, Nield J, Hippler M (2010) The composition and structure of photosystem I-associated antenna from Cyanidioschyzon merolae. Plant J 62:886–897
Chen JP, Tai CY, Chen BH (2004) Improved liquid chromatographic method for determination of carotenoids in Taiwanese mango (Mangifera indica L.). J Chromatogr A 1054:261–268
Croce R, van Amerongen H (2013) Light-harvesting in photosystem I. Photosynth Res 116:153–166
Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR (2004) The evolution of modern eukaryotic phytoplankton. Science 305:354–360
Fromme P, Jordan P, Krauß N (2001) Structure of photosystem I. Biochim Biophys Acta, Bioenerg 1507:5–31
Gardian Z, Bumba L, Schrofel A, Herbstova M, Nebesarova J, Vacha F (2007) Organisation of photosystem I and photosystem II in red alga Cyanidium caldarium: encounter of cyanobacterial and higher plant concepts. Biochim Biophys Acta, Bioenerg 1767:725–731
Giera W, Szewczyk S, McConnell MD, Redding KE, van Grondelle R, Gibasiewicz K (2018) Uphill energy transfer in photosystem I from Chlamydomonas reinhardtii. Time-resolved fluorescence measurements at 77 K. Photosynth Res 137:321–335
Golbeck JH (1992) Structure and function of photosystem I. Ann Rev Plant Physiol Plant Mol Biol 43:293–324
Hamada F, Murakami A, Akimoto S (2017) Adaptation of divinyl chlorophyll a/b-containing cyanobacterium to different light conditions: three strains of Prochlorococcus marinus. J Phys Chem B 121:9081–9090
Haniewicz P, Abram M, Nosek L, Kirkpatrick J, El-Mohsnawy E, Janna Olmos JD, Kouŕil R, Kargul JM (2018) Molecular mechanisms of photoadaptation of photosystem I supercomplex of in an evolutionary cyanobacterial/algal intermediate. Plant Physiol 176:1433–1451
Haworth P, Watson JL, Arntzen CJ (1983) The detection, isolation and characterization of a light-harvesting complex which is specifically associated with photosystem I. Biochim Biophys Acta, Bioenerg 724:151–158
Hippler M, Nelson N (2021) The plasticity of photosystem I. Plant Cell Physiol 62:1073–1081
Ikeuchi M, Inoue Y (1988) A new photosystem II reaction center component (4.8 kDa protein) encoded by chloroplast genome. FEBS Lett 241:99–104
Kato K, Nagao R, Ueno Y, Yokono M, Suzuki T, Jiang TY, Dohmae N, Akita F, Akimoto S, Miyazaki N, Shen J-R (2022) Structure of a tetrameric photosystem I from a glaucophyte alga Cyanophora paradoxa. Nat Commun 13:1679
Liu S-L, Chiang Y-R, Yoon HS, Fu H-Y (2020) Comparative genome analysis reveals Cyanidiococcus gen. nov., a new extremophilic red algal genus sister to Cyanidioschyzon (Cyanidioschyzonaceae, Rhodophyta). J Phycol 56:1428–1442
Nagao R (2022) Handbook of cyanobacterial PSI structures. [Kindle edition]. Retrieved from Amazon.com. https://www.amazon.co.jp/Handbook-Cyanobacterial-PSI-Structures-English-ebook/dp/B09Z8R7B4K
Nagao R, Yokono M, Akimoto S, Tomo T (2013) High excitation energy quenching in fucoxanthin chlorophyll a/c-binding protein complexes from the diatom Chaetoceros gracilis. J Phys Chem B 117:6888–6895
Nagao R, Yamaguchi M, Nakamura S, Ueoka-Nakanishi H, Noguchi T (2017) Genetically introduced hydrogen bond interactions reveal an asymmetric charge distribution on the radical cation of the special-pair chlorophyll P680. J Biol Chem 292:7474–7486
Nagao R, Kagatani K, Ueno Y, Shen J-R, Akimoto S (2019a) Ultrafast excitation energy dynamics in a diatom photosystem I-antenna complex: a femtosecond fluorescence upconversion study. J Phys Chem B 123:2673–2678
Nagao R, Yokono M, Ueno Y, Shen J-R, Akimoto S (2019b) Low-energy chlorophylls in fucoxanthin chlorophyll a/c-binding protein conduct excitation energy transfer to photosystem I in diatoms. J Phys Chem B 123:66–70
Nagao R, Yokono M, Ueno Y, Shen J-R, Akimoto S (2019c) pH-sensing machinery of excitation energy transfer in diatom PSI-FCPI complexes. J Phys Chem Lett 10:3531–3535
Nagao R, Kato K, Ifuku K, Suzuki T, Kumazawa M, Uchiyama I, Kashino Y, Dohmae N, Akimoto S, Shen J-R, Miyazaki N, Akita F (2020a) Structural basis for assembly and function of a diatom photosystem I-light-harvesting supercomplex. Nat Commun 11:2481
Nagao R, Ueno Y, Akimoto S, Shen J-R (2020b) Effects of CO2 and temperature on photosynthetic performance in the diatom Chaetoceros gracilis. Photosynth Res 146:189–195
Nagao R, Yokono M, Ueno Y, Shen J-R, Akimoto S (2020c) Excitation-energy transfer and quenching in diatom PSI-FCPI upon P700 cation formation. J Phys Chem B 124:1481–1486
Nelson N, Junge W (2015) Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu Rev Biochem 84:659–683
Nikolova D, Weber D, Scholz M, Bald T, Scharsack JP, Hippler M (2017) Temperature-induced remodeling of the photosynthetic machinery tunes photosynthesis in the thermophilic alga Cyanidioschyzon merolae. Plant Physiol 174:35–46
Pi X, Tian L, Dai H-E, Qin X, Cheng L, Kuang T, Sui S-F, Shen J-R (2018) Unique organization of photosystem I-light-harvesting supercomplex revealed by cryo-EM from a red alga. Proc Natl Acad Sci U S A 115:4423–4428
Polívka T, Sundström V (2004) Ultrafast dynamics of carotenoid excited states−From solution to natural and artificial systems. Chem Rev 104:2021–2072
Shen J-R (2022) Structure, function, and variations of the photosystem I-antenna supercomplex from different photosynthetic organisms. In: Harris JR, Marles-Wright J (eds) Macromolecular protein complexes IV. Subcellular biochemistry, vol 99. Springer, Cham, pp 351–377
Tan S, Wolfe GR, Cunningham FX Jr, Gantt E (1995) Decrease of polypeptides in the PS I antenna complex with increasing growth irradiance in t the red alga Porphyridium cruentum. Photosynth Res 45:1–10
Thangaraj B, Jolley CC, Sarrou I, Bultema JB, Greyslak J, Whitelegge JP, Lin S, Kouřil R, Subramanyam R, Boekema EJ, Fromme P (2011) Efficient light harvesting in a dark, hot, acidic environment: the structure and function of PSI-LHCI from Galdieria sulphuraria. Biophys J 100:135–143
Tian L, Liu Z, Wang F, Shen L, Chen J, Chang L, Zhao S, Han G, Wang W, Kuang T, Qin X, Shen J-R (2017) Isolation and characterization of PSI-LHCI super-complex and their sub-complexes from a red alga Cyanidioschyzon merolae. Photosynth Res 133:201–214
Watanabe M, Kubota H, Wada H, Narikawa R, Ikeuchi M (2011) Novel supercomplex organization of photosystem I in Anabaena and Cyanophora paradoxa. Plant Cell Physiol 52:162–168
Wientjes E, van Stokkum IHM, van Amerongen H, Croce R (2011) The role of the individual Lhcas in photosystem I excitation energy trapping. Biophys J 101:745–754
Wolfe GR, Cunningham FX Jr, Grabowski B, Gantt E (1994) Isolation and characterization of Photosystems I and II from the red alga Porphyridium cruentum. Biochim Biophys Acta, Bioenerg 1188:357–366
Xu C, Pi X, Huang Y, Han G, Chen X, Qin X, Huang G, Zhao S, Yang Y, Kuang T, Wang W, Sui S-F, Shen J-R (2020) Structural basis for energy transfer in a huge diatom PSI-FCPI supercomplex. Nat Commun 11:5081
Yoon HS, Zuccarello GC, Bhattacharya D (2010) Evolutionary history and taxonomy of red algae. In: Seckbach J, Chapman DJ (eds) Red algae in the genomic age. Springer, Dordrecht, pp 25–42
Zapata M, Rodríguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar Ecol Prog Ser 195:29–45
Acknowledgements
We thank Ms. Kumiyo Kato for her assistance in this study. This work was supported by JSPS KAKENHI grant Nos. JP21K19085 (R.N.), JP20H02914 (K.K.), and JP17H06434 and JP22H04916 (J.-R.S.).
Funding
This study was supported by Japan Society for the Promotion of Science (Grant Nos. JP21K19085, JP20H02914, JP17H06434, JP22H04916).
Author information
Authors and Affiliations
Contributions
R.N. conceived the project; R.N. purified the three types of preparations and analyzed their biochemical characterization; R.N. measured absorption and fluorescence spectra; Y.U. measured time-resolved fluorescence; M.F. and S.A. analyzed time-resolved fluorescence data; R.N., K.K., J.-R.S., and S.A. provided experimental and funding resources; and R.N. and S.A. wrote the manuscript, and all of the authors joined the discussion of the results.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagao, R., Ueno, Y., Furutani, M. et al. Biochemical and spectroscopic characterization of PSI-LHCI from the red alga Cyanidium caldarium. Photosynth Res 156, 315–323 (2023). https://doi.org/10.1007/s11120-023-00999-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-023-00999-y