Abstract
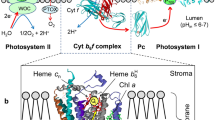
Although there is an extensive literature on the properties and possible electron transfer pathways of cytochrome b-559, which is a prominent subunit of the multi-subunit photosystem II complex which functions in oxygenic photosynthesis, there is presently no consensus on the function of b-559 in the photosynthetic electron transport chain. The inability in earlier times to define a redox-linked function of this cytochrome was, to a large extent, a consequence of an absence of biochemical and structure information to complement an extensive array of spectrophotometric studies of the cytochrome in situ. Based on the location of hetero-dimeric b-559 in the photosystem II reaction center complex, derived from crystal crystallographic structure analysis, and the absence of a necessary redox function for the cytochrome in PSII, it is proposed that the main function of cytochrome b-559 is linked to its role as a structure component in the PSII reaction center complex. This function resides in the association of b-559 through its heme histidine residues in the trans-membrane domains of the PsbE and PsbF subunits of the PSII reaction center. These subunits, along with PsbJ, are inferred, from the analysis of structure, to define the intra-membrane portal in the PSII reaction center for plastoquinol (PQH2) export which, through the PSII complex, provides the redox link to the cytochrome b6f complex in the electron transfer chain.


Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine; cyt, cytochrome
- mDa:
-
Megadalton
- ms:
-
Millisecond
- OEC:
-
Oxygen-evolving complex
- PQ:
-
Plastoquinone
- p, n :
-
Electrochemically positive and negative sides of the membrane
- PS:
-
Photosystem
- Psb:
-
An acronym for photosystem II
References
Babcock GT, Widger WR, Cramer WA, Oertling WA, Metz JG (1985) Axial ligands of cytochrome b-559: identification and requirement for a heme-cross-linked polypeptide structure. Biochemistry 24:3638–3645
Boardman NK, Anderson J (1967) Fractionation of the photochemical systems of photosynthesis. II. cytochrome and carotenoid contents of particles isolated from spinach chloroplasts. Biochim Biophys Acta 143:187–203
Böhme H, Cramer WA (1971) Plastoquinone mediates electron transport between cytochrome b-559 and cytochrome f in spinach chloroplasts. FEBS Lett 15:349–435
Chance B (1982) Structure and function of the redox site of cytochrome oxidase. Adv Exp Med Biol 148:95–109
Chu H-A, Chen Y-H (2016) The roles of cytochrome b-559 in assembly and photoprotection of photosystem II revealed by site-directed mutagenesis studies. Front Plant Sci 6:1261
Cramer WA, Butler WL (1967) Light induced absorption changes of twocytochrome b components in the electron transport chain of spinach chloroplasts. Biochim Biophys Acta 14:332–339
Cramer, W. A., and D. B. Knaff (1991) Oxidation-Reduction; Electron and Proton Transfer. Energy Transduction in Biological Membranes; Chapter 2; Springer Study Edition [paperback, ISBN 0–387–97533–0]; 579 pp.
Cramer WA, Tae G-S, Furbacher P, Bottger M (1993) The enigmatic cytochrome b-559 of oxygenic photosynthesis. Physiol Plant 88:705–711
Crofts AR, Berry EA (1998) Structure and function of the cytochrome bc1 complex of mitochondria and photosynthetic bacteria. Curr Opin Struct Biol 8:501–509
Deisenhofer J, Michel H (1989) The photosynthetic reaction center from the purple photosynthetic bacterium, Rhodopseudomonas viridis. Science 245:1463–1473
Van Eerden FJ, Melo MN, Frederix PWJM, Periole X, Marrink SJ (2017) Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. Nature Comm 8:15214–15221
Horton P, Cramer WA (1974) The accessibility of the chloroplast cytochromes f and b-559 to ferricyanide. Biochim Biophys Acta 368:348–360
Horton P, Cramer WA (1975) Acid-base induced redox changes of the chloroplast b-559. FEBS Lett 56:244–247
Horton P, Whitmarsh J, Cramer WA (1976) On the specific site of action of 3-(3,4 di-chlorophenyl)-1,1-dimethylurea in chloroplasts: Inhibition of a dark acid-induced decrease in midpoint potential of cytochrome b-559. Arch Biochem Biophys 17:519–524
Kaminskaya O, Shuvalov VA, Renger G (2007) Evidence for a novel quinone binding site in the photosystem II (PS II) complex that regulates the redox potential of cytochrome b559. Biochemistry 46:1091–1105
Kern J et al (2018) Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563:421–425
Knaff DB, Arnon DI (1969) Light-induced photo-oxidation of a chloroplast b-type cytochrome at -189 C. Proc. Natl Acad Sci. U S 63:956–962
Lundegarth H (1962) Quantitative relations between chlorophyll and cytochromes in chloroplasts. Physiol Plantarum 15:390
McKenzie SD, Ibrahim IM, Aryal UK, Puthiyaveetil S (2020) Stoichiometry of protein complexes in plant photosynthetic membranes. Biochim Biophys Acta 2:148141
Müh F, Zouni A (2016) Cytochrome b559 in Photosystem II. In: Cramer WA, Kallas T (eds) Cytochrome complexes, evolution, structures, energy transduction, and signaling advances in photosynthesis and respiration, vol 41. Springer, Dordrecht, pp 143–175
Pakrasi HB, De Ciechi P, Whitmarsh J (1991) Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. The EMBO J 10:1619–1627
Pospisil P (2011) Enzymatic function of cytochrome b-559 in photosystem II. Photochem Photobiol B 104:141–147
Slater EC (1967) An evaluation of the Mitchell hypothesis of chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Eur J Biochem 1:317–326
Tae G-S, Everly RM, Cramer WA, Madgwick SA, Rich PR (1993) On the question of the identity of cytochrome b-560 in thylakoid stromal membranes. Photosyn Res 36:141–146
Thompson LK, Brudvig GW (1988) Cytochrome b-559 may function to protect photosystem II from photoinhibition. Biochemistry 27:6653–6658
Whitmarsh J, Cramer WA (1978) A pathway for the reduction of cytochrome b-559 by photosystem II in chloroplasts. Biochim Biophys Acta 501:83–93
Acknowledgements
The authors’ research on ‘Structure-Function of Cytochromes in Oxygenic Photosynthesis,’ was supported at different times by non-overlapping grants from the Fogarty Foundation (TW0-1235), the National Science Foundation (GB-26635), the National Institutes of Health (GM-18457), a Research Career Development Award from the NIH (1 KO4 GM-29735), and grants from the USDA (59-2182-1-1-683-0) and DOE (Dept. of Energy) grant DE-SC0018238. We thank R. Harding for assistance with the assembly of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cramer, W.A., Zakharov, S.D. Concerning the enigmatic cytochrome b-559 of oxygenic photosynthesis. Photosynth Res 153, 157–162 (2022). https://doi.org/10.1007/s11120-022-00936-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00936-5