Abstract
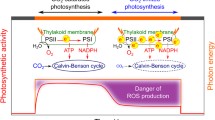
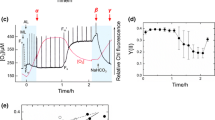
Oxygenic photosynthesis converts light energy into chemical energy via electron transport and assimilates CO2 in the Calvin–Benson cycle with the chemical energy. Thus, high light and low CO2 conditions induce the accumulation of electrons in the photosynthetic electron transport system, resulting in the formation of reactive oxygen species. To prevent the accumulation of electrons, oxygenic photosynthetic organisms have developed photoprotection mechanisms, including non-photochemical quenching (NPQ) and alternative electron flow (AEF). There are diverse molecular mechanisms underlying NPQ and AEF, and the corresponding molecular actors have been identified and characterized using a model green alga Chlamydomonas reinhardtii. In contrast, detailed information about the photoprotection mechanisms is lacking for other green algal species. In the current study, we examined the photoprotection mechanisms responsive to CO2 in the green alga Chlorella variabilis by combining the analyses of pulse-amplitude-modulated fluorescence, O2 evolution, and the steady-state and time-resolved fluorescence spectra. Under the CO2-limited condition, ΔpH-dependent NPQ occurred in photosystems I and II. Moreover, O2-dependent AEF was also induced. Under the CO2-limited condition with carbon supplementation, NPQ was relaxed and light-harvesting chlorophyll-protein complex II was isolated from both photosystems. In C. variabilis, the O2-dependent AEF and the mechanisms that instantly convert the light-harvesting functions of both photosystems may be important for maintaining efficient photosynthetic activities under various CO2 conditions.






Similar content being viewed by others
Abbreviations
- AEF:
-
Alternative electron flow
- AFI:
-
Absolute fluorescence intensity
- AL:
-
Actinic light
- Chl:
-
Chlorophyll
- Cyt:
-
Cytochrome
- EET:
-
Excitation energy transfer
- FDA:
-
Fluorescence decay-associated
- FLV:
-
Flavodiiron
- LHC:
-
Light-harvesting chlorophyll-protein complex
- LHCSR:
-
Light-harvesting complex stress-related
- ML:
-
Measuring light
- NPQ:
-
Non-photochemical quenching
- PAM:
-
Pulse-amplitude modulated
- PET:
-
Photosynthetic electron transport
- PS:
-
Photosystem
- PTOX:
-
Plastid terminal oxidase
- PQ:
-
Plastoquinone
- RC:
-
Reaction center
- Rubisco:
-
Ribulose 1,5-bisphosphate carboxylase/oxygenase
- TRF:
-
Time-resolved fluorescence
References
Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A (2012) Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1817:1483–1489
Albanese P, Manfredi M, Meneghesso A, Marengo E, Saracco G, Barber J, Morosinotto T, Pagliano C (2016) Dynamic reorganization of photosystem II supercomplexes in response to variations in light intensities. Biochim Biophys Acta 1857:1651–1660
Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T (2010) Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc Natl Acad Sci USA 107:11128–11133
Allahverdiyeva Y, Isojärvi J, Zhang P, Aro EM (2015) Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life 5:716–743
Allorent G, Tokutsu R, Roach T, Peers G, Cardol P, Girard-Bascou J, Seigneurin-Berny D, Petroutsos D, Kuntz M, Breyton C, Franck F, Wollman FA, Niyogi KK, Krieger-Liszkay A, Minagawa J, Finazzi G (2013) A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25:545–557
Allorent G, Lefebvre-Legendre L, Chappuis R, Kuntz M, Truong TB, Niyogi KK, Ulm R, Goldschmidt-Clermont M (2016) UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 113:14864–14869
Alric J, Johnson X (2017) Alternative electron transport pathways in photosynthesis: a confluence of regulation. Curr Opin Plant Biol 37:78–86
Andrizhiyevskaya EG, Chojnicka A, Bautista JA, Diner BA, van Grondelle R, Dekker JP (2005) Origin of the F685 and F695 fluorescence in photosystem II. Photosynth Res 84:173–180
Ballottari M, Truong TB, De Re E, Erickson E, Stella GR, Fleming GR, Bassi R, Niyogi KK (2016) Identification of pH-sensing sites in the light harvesting complex stress-related 3 protein essential for triggering non-photochemical quenching in Chlamydomonas reinhardtii. J Biol Chem 291:7334–7346
Bauwe H, Hegemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15:330–336
Blankenship RE (2014) Molecular mechanisms of photosynthesis, 2nd edn. Wiley-Blackwell, Hoboken
Bonente G, Passarini F, Cazzaniga S, Mancone C, Buia MC, Tripodi M, Bassi R, Caffarri S (2008) The occurrence of the psbS gene product in Chlamydomonas reinhardtii and in other photosynthetic organisms and its correlation with energy quenching. Photochem Photobiol 84:1359–13703
Cardol P, Forti G, Finazzi G (2011) Regulation of electron transport in microalgae. Biochim Biophys Acta 1807:912–918
Chaux F, Peltier G, Johnson X (2015) A security network in PSI photoprotection: regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front Plant Sci 6:875
Chaux F, Burlacot A, Mekhalfi M, Auroy P, Blangy S, Richaud P, Peltier G (2017) Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant Physiol 174:1825–1836
Chmeliov J, Gelzinis A, Songaila E, Augulis R, Duffy CD, Ruban AV, Valkunas L (2016) The nature of self-regulation in photosynthetic light-harvesting antenna. Nat Plants 2:16045
Correa-Galvis V, Poschmann G, Melzer M, Stühler K, Jahns P (2016) PsbS interactions involved in the activation of energy dissipation in Arabidopsis. Nat Plants 2:15225
Croce R, van Amerongen H (2014) Natural strategies for photosynthetic light harvesting. Nat Chem Biol 10:492–501
Derks A, Schaven K, Bruce D (2015) Diverse mechanisms for photoprotection in photosynthesis: dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim Biophys Acta 1847:468–485
Dinc E, Tian L, Roy LM, Roth R, Goodenough U, Croce R (2016) LHCSR1 induces a fast and reversible pH-dependent fluorescence quenching in LHCII in Chlamydomonas reinhardtii cells. Proc Natl Acad Sci USA 113:7673–7678
Garnier J, Maroc J, Guyon D (1986) Low-temperature fluorescence emission spectra and chlorophyll-protein complexes in mutants of Chlamydomonas reinhardtii: evidence for a new chlorophyll-a-protein complex related to Photosystem I. Biochim Biophys Acta 851:395–406
Gerotto C, Morosinotto T (2013) Evolution of photoprotection mechanisms upon land colonization: Evidence of PSBS-dependent NPQ in late Streptophyte algae. Physiol Plant 149:583–598
Gerotto C, Alboresi A, Meneghesso A, Jokel M, Suorsa M, Aro EM, Morosinotto T (2016) Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc Natl Acad Sci USA 113:12322–12327
Girolomoni L, Cazzaniga S, Pinnola A, Perozeni F, Ballottari M, Bassi R (2019) LHCSR3 is a nonphotochemical quencher of both photosystems in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 116:4212–4217
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Grimme LH, Boardman NK (1972) Photochemical activities of a particle fraction P1 obtained from the green alga Chlorella fuska. Biochem Biophys Res Commun 49:1617–1623
Hanawa H, Ishizaki K, Nohira K, Takagi D, Shimakawa G, Sejima T, Shaku K, Makino A, Miyake C (2017) Land plants drive photorespiration as higher electron-sink: Comparative study of post-illumination transient O2-uptake rates from liverworts to angiosperms through ferns and gymnosperms. Physiol Plant 161:138–149
Hayashi R, Shimakawa G, Shaku K, Shimizu S, Akimoto S, Yamamoto H, Amako K, Sugimoto T, Tamoi M, Makino A, Miyake C (2014) O2-dependent large electron flow functioned as an electron sink, replacing the steady-state electron flux in photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803, but not in the cyanobacterium Synechococcus sp. PCC 7942. Biosci Biotechnol Biochem 78:384–393
Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49:511–523
Ichimura T (1971) Sexual cell division and conjugation-papilla formation in sexual reproduction of Closterium strigosum. In: Nishizawa K (ed) Proceedings of the 7th international seaweed symposium, University of Tokyo Press, Tokyo, pp 208–214
Ihalainen JA, van Stokkum IHM, Gibasiewicz K, Germano M, van Grondelle R, Dekker JP (2005) Kinetics of excitation trapping in intact photosystem I of Chlamydomonas reinhardtii and Arabidopsis thaliana. Biochim Biophys Acta 1706:267–275
Ihnken S, Kromkamp JC, Beardall J, Silsbe GM (2014) State-transitions facilitate robust quantum yields and cause an over-estimation of electron transport in Dunaliella tertiolecta cells held at the CO2 compensation point and re-supplied with DIC. Photosynth Res 119:257–272
Iwai M, Kato N, Minagawa J (2007) Distinct physiological responses to a high light and low CO2 environment revealed by fluorescence quenching in photoautotrophically grown Chlamydomonas reinhardtii. Photosynth Res 94:307–314
Jokel M, Johnson X, Peltier G, Aro EM, Allahverdiyeva Y (2018) Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant J 94:822–835
Jordan DB, Ogren WL (1981) Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature 291:513–515
Kim E, Akimoto S, Tokutsu R, Yokono M, Minagawa J (2017) Fluorescence lifetime analyses reveal how the high light-responsive protein LHCSR3 transforms PSII light-harvesting complexes into an energy-dissipative state. J Biol Chem 292:18951–18960
Kirilovsky D (2015) Modulating energy arriving at photochemical reaction centers: orange carotenoid protein-related photoprotection and state transitions. Photosynth Res 126:3–17
Kosuge K, Tokutsu R, Kim E, Akimoto S, Yokono M, Ueno Y, Minagawa J (2018) LHCSR1-dependent fluorescence quenching is mediated by excitation energy transfer from LHCII to photosystem I in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 115:3722–3727
Kozaki A, Takebe G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Krieger-Liszkay A, Feilke K (2016) The dual role of the plastid terminal oxidase PTOX: Between a protective and a pro-oxidant function. Front Plant Sci 6:1147
Lea-Smith DJ, Bombelli P, Howe VR (2016) Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochim Biophys Acta 1857:247–255
Li X, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Miller AG, Espie GS, Bruce D (1996) Characterization of the non-photochemical quenching of chlorophyll fluorescence that occurs during the active accumulation of inorganic carbon in the cyanobacterium Synechococcus PCC 7942. Photosynth Res 49:251–262
Mimuro M, Akimoto S, Tomo T, Yokono M, Miyashita H, Tsuchiya T (2007) Delayed fluorescence observed in the nanosecond time region at 77 K originates directly from the photosystem II reaction center. Biochim Biophys Acta 1767:327–334
Mirkovic T, Ostroumov EE, Anna JM, van Grondelle R, Govindjee, Scholes GD (2017) Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem Rev 117:249–293
Miyake C (2010) Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: Molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Mullineaux CW (2014) Co-existence of photosynthetic and respiratory activities in cyanobacterial thylakoid membranes. Biochim Biophys Acta 1837:503–511
Mullineaux CW, Emlyn-Jones D (2005) State transitions: an example of acclimation to low-light stress. J Exp Bot 56:389–393
Palmqvist K, Sundblad G, Wingsle G, Samuelsson G (1990) Acclimation of photosynthetic light reactions during induction of inorganic carbon accumulation in the green alga Chlamydomonas reinhardtii. Plant Physiol 94:357–366
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–521
Petroutsos D, Tokutsu R, Maruyama S, Flori S, Greiner A, Magneschi L, Cusant L, Kottke T, Mittag M, Hegemann P, Finazzi G, Minagawa J (2016) A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 537:563–566
Pinnola A, Cazzaniga S, Alboresi A, Nevo R, Levin-Zaidman S, Reich Z, Bassi R (2015) Light-harvesting complex stress-related proteins catalyze excess energy dissipation in both photosystems of Physcomitrella patens. Plant Cell 27:3213–3227
Romão CV, Vicente JB, Borges PT, Frazão C, Teixeira M (2016) The dual function of flavodiiron proteins: oxygen and/or nitric oxide reductases. J Biol Inorg Chem 21:39–52
Saroussi S, Karns DAJ, Thomas DC, Bloszies C, Fiehn O, Posewitz MC, Grossman AR (2019) Alternative outlets for sustaining photosynthetic electron transport during dark-to-light transitions. Proc Natl Acad Sci USA 116:11518–11527
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Shaku K, Shimakawa G, Hashiguchi M, Miyake C (2016) Reduction-induced suppression of electron flow (RISE) in the photosynthetic electron transport system of Synechococcus elongatus PCC 7942. Plant Cell Physiol 57:1443–1453
Shibata Y, Nishi S, Kawakami K, Shen JR, Renger T (2013) Photosystem II does not possess a simple excitation energy funnel: time-resolved fluorescence spectroscopy meets theory. J Am Chem Soc 135:6903–6914
Shikanai T (2016) Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I. Photosynth Res 129:253–260
Shimakawa G, Shaku K, Nishi A, Hayashi R, Yamamoto H, Sakamoto K, Makino A, Miyake C (2015) FLAVODIIRON2 and FLAVODIIRON4 proteins mediate and oxygen-dependent alternative electron flow in Synechocystis sp. PCC 6803 under CO2-limited conditions. Plant Physiol 167:472–480
Shimakawa G, Akimoto S, Ueno Y, Wada A, Shaku K, Takahashi Y, Miyake C (2016a) Diversity in photosynthetic electron transport under [CO2]-limitation: the cyanobacterium Synechococcus sp. PCC 7002 and green alga Chlamydomonas reinhardtii drive an O2-dependent alternative electron flow and non-photochemical quenching of chlorophyll fluorescence during CO2-limited photosynthesis. Photosynth Res 130:293–305
Shimakawa G, Shaku K, Miyake C (2016b) Oxidation of P700 in photosystem I is essential for the growth of cyanobacteria. Plant Physiol 172:1443–1450
Shimakawa G, Ishizaki K, Tsukamoto S, Tanaka M, Sejima T, Miyake C (2017a) The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant Physiol 173:1636–1647
Shimakawa G, Matsuda Y, Nakajima K, Tamoi M, Shigeoka S, Miyake C (2017b) Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci Rep 7:41022
Sültemeyer DF, Miller AG, Espie GS, Fock HP, Canvin DT (1989) Active CO2 transport by the green alga Chlamydomonas reinhardtii. Plant Physiol 89:1213–1219
Tokutsu R, Minagawa J (2013) Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 110:10016–10021
Ueno Y, Shimakawa G, Miyake C, Akimoto S (2018) Light-harvesting strategy during CO2-dependent photosynthesis in the green alga Chlamydomonas reinhardtii. J Phys Chem Lett 9:1028–1033
Ueno Y, Aikawa S, Kondo A, Akimoto S (2019) Adaptation of light-harvesting functions of unicellular green algae to different light qualities. Photosynth Res 139:145–154
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
van Thor JJ, Mullineaux CW, Matthijs HCP, Hellingwerf KJ (1998) Light harvesting and state transitions in cyanobacteria. Bot Acta 111:430–443
Wlodarczyk LM, Dinc E, Croce R, Dekker JP (2016) Excitation energy transfer in Chlamydomonas reinhardtii deficient in the PSI core or the PSII core under conditions mimicking state transitions. Biochim Biophys Acta 1857:625–633
Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20:3623–3630
Yokono M, Murakami A, Akimoto S (2011) Excitation energy transfer between photosystem II and photosystem I in red algae: larger amounts of phycobilisome enhance spillover. Biochim Biophys Acta 1807:847–853
Yokono M, Takabayashi A, Akimoto S, Tanaka A (2015) A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun 6:6675
Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM (2009) Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4:e5331
Acknowledgements
This work was supported by JSPS KAKENHI (Grant No. 18J10095 to Y.U., No. 16J03443 to G.S., and No. 16H06553 to S.A.) and JST CREST (Grant No. JPMJCR15O3 to C.M.). We thank Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ueno, Y., Shimakawa, G., Aikawa, S. et al. Photoprotection mechanisms under different CO2 regimes during photosynthesis in a green alga Chlorella variabilis. Photosynth Res 144, 397–407 (2020). https://doi.org/10.1007/s11120-020-00757-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-020-00757-4