Abstract
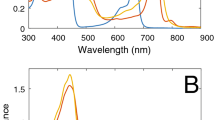
Chlorosomes of green photosynthetic bacteria are the most amazing example of long-range ordered natural light-harvesting antennae. Chlorosomes are the largest among all known photosynthetic light-harvesting structures (~ 104–105 pigments in the aggregated state). The chlorosomal bacteriochlorophyll (BChl) c/d/e molecules are organized via self-assembly and do not require proteins to provide a scaffold for efficient light harvesting. Despite numerous investigations, a consensus regarding the spatial structure of chlorosomal antennae has not yet been reached. In the present work, we studied hyperchromism/hypochromism in the chlorosomal BChl c Q/B absorption bands of the green photosynthetic bacterium Chloroflexus (Cfx.) aurantiacus. The chlorosomes were isolated from cells grown under different light intensities and therefore, as we discovered earlier, they had different sizes of both BChl c antennae and their unit building blocks. We have shown experimentally that the Q-/B-band hyperchromism/hypochromism is proportional to the size of the chlorosomal antenna. We explained theoretically these findings in terms of excitonic intensity borrowing between the Q and B bands for the J-/H-aggregates of the BChls. The theory developed by Gülen (Photosynth Res 87:205–214, 2006) showed the dependence of the Q-/B-band hyperchromism/hypochromism on the structure of the aggregates. For the model of exciton-coupled BChl c linear chains within a unit building block, the theory predicted an increase in the hyperchromism/hypochromism with the increase in the number of molecules per chain and a decrease in it with the increase in the number of chains. It was previously shown that this model ensured a good fit with spectroscopy experiments and approximated the BChl c low packing density in vivo. The presented experimental and theoretical studies of the Q-/B-band hyperchromism/hypochromism permitted us to conclude that the unit building block of Cfx. aurantiacus chlorosomes comprises of several short BChl c chains.
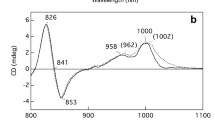
This conclusion is in accordance with previous linear and nonlinear spectroscopy studies on Cfx. aurantiacus chlorosomes.











Similar content being viewed by others
Abbreviations
- BChl:
-
Bacteriochlorophyll
- Cfx :
-
Chloroflexus
- CMC:
-
Chlorosome-membrane complexes
- Cba :
-
Chlorobaculum
- OD:
-
Optical density
References
Arellano J, Melo T, Borrego C, Garcia-Gil J, Naqvi K (2000) Nanosecond laser photolysis studies of chlorosomes and artificial aggregates containing bacteriochlorophyll e: evidence for the proximity of carotenoids and bacteriochlorophyll a in chlorosomes from Chlorobium phaeobacteroides strain CL1401. Photochem Photobiol 72:669–675
Carbonera D, Bordignon E, Giacometti G, Agostini G, Vianelli A, Vannini C (2001) Fluorescence and absorption detected magnetic resonance of chlorosomes from green bacteria Chlorobium tepidum and Chloroflexus aurantiacus. A comparative study. J Phys Chem B 105:246–255
Castenholz RW (1969) Thermophilic blue-green algae and the thermal environment. Bacteriol Rev 33:476–504
Clayton RK (1980) Photosynthesis: physical mechanisms and chemical patterns. Cambridge University Press, Cambridge
DeVoe H, Tinoco I Jr (1962) The hypochromism of helical polynucleotides. J Mol Biol 4:518–527
Didraga C, Knoester J (2003) Absorption and dichroism spectra of cylindrical J aggregates and chlorosomes of green bacteria. J Lumin 102:60–66
Dracheva T, Taisova A, Fetisova Z (1998) Circular dichroism spectroscopy as a test for the chlorosome antenna structure. In: Garab G (ed) Photosynthesis: mechanisms and effects, vol 1. Kluwer Academic Publishers, Dordrecht, pp 129–132
Egawa A, Fujiwara T, Mizoguchi T, Kakitani Y, Koyama Y, Akutsu H (2007) Structure of the light-harvesting bacteriochlorophyll c assembly in chlorosomes from Chlorobium limicola determined by solid-state NMR. Proc Natl Acad Sci USA 104(3):790–795
Fetisova Z (2004) Survival strategy of photosynthetic organisms. 1. Variability of the extent of light-harvesting pigment aggregation as a structural factor optimizing the function of oligomeric photosynthetic antenna model calculations. Mol Biol (Mosk) 38:434–440
Fetisova Z, Fok M (1984) Optimization routes for the transformation of light energy in primary acts of photosynthesis. I. The necessity of structure optimization for photosynthetic unit and method for the calculation of its efficiency. Mol Biol (Mosk) 18:1354–1359
Fetisova Z, Kharchenko S, Abdourakhmanov I (1986) Strong orientational ordering of the near-infrared transition moment vectors of light-harvesting antenna bacterioviridin in chromatophores of the green photosynthetic bacterium Chlorobium limicola. FEBS Lett 199:234–236
Fetisova Z, Freiberg A, Timpmann K (1988) Long-range molecular order as an efficient strategy for light harvesting in photosynthesis. Nature (London) 334:633–634
Fetisova Z, Shibaeva L, Fok M (1989) Biological expedience of oligomerization of chlorophyllous pigments in natural photosynthetic systems. J Theor Biol 140:167–184
Fetisova Z, Mauring K (1992) Experimental evidence of oligomeric organization of antenna bacteriochlorophyll c in green bacterium Chloroflexus aurantiacus by spectral hole burning. FEBS Lett 307:371–374
Fetisova Z, Mauring K (1993) Spectral hole burning study of intact cells of green bacterium Chlorobium limicola. FEBS Lett 323:159–162
Fetisova Z, Mauring K, Taisova A (1994) Strongly exciton coupled BChl e chromophore system in chlorosomal antenna of intact cells of green bacterium Chlorobium phaeovibrioides: A spectral hole burning study. Photosynth Res 41:205–210
Fetisova Z, Freiberg A, Mauring K, Novoderezhkin V, Taisova A, Timpmann K (1996) Excitation energy transfer in chlorosomes of green bacteria: theoretical and experimental studies. Biophys J 71:101–995
Frigaard N-U, Bryant DA (2004) Seeing green bacteria in a new light: genomics-enabled studies of the photosynthetic apparatus in green sulfur bacteria and filamentous anoxygenic phototrophic bacteria. Arch Microbiol 182:265–276
Frigaard N-U, Bryant D (2006) Chlorosomes: antenna organelles in green photosynthetic bacteria. In: Shively JM (ed) Complex intracellular structures in prokaryotes Microbiology monographs, vol 2. Springer, Berlin, pp 79–114
Furumaki S, Vacha F, Habuchi S, Tsukatani Y, Bryant D, Vacha M (2011) Absorption linear dichroism measured directly on a single light-harvesting system: the role of disorder in chlorosomes of green photosynthetic bacteria. J Am Chem Soc 133(17):6703–6710
Furumaki S, Yabiku Y, Habuchi S, Tsukatani Y, Bryant D, Vacha M (2012) Circular dichroism measured on single chlorosomal light-harvesting complexes of green photosynthetic bacteria. J Phys Chem Lett 3:3545–3549
Ganapathy S, Oostergetel G, Wawrzyniak P, Reus M, Gomez Maqueo Chew A, Buda F, Boekema E, Bryant D, Holzwarth A, de Groot H (2009) Alternating syn-anti bacteriochlorophylls form concentric helical nanotubes in chlorosomes. Proc Natl Acad Sci USA 106:8525–8530
Gerola P, Olson J (1986) A new bacteriochlorophyll a-protein complex associated with chlorosomes of green sulfur bacteria. Biochim Biophys Acta 848:69–76
Golecki J, Oelze J (1987) Quantitative relationship between bactenochlorophyll content, cytoplasmic membrane structure and chlorosome size in Chloroflexus aurantiacus. Arch Microbiol 148:236–241
Gomez Maqueo Chew A, Frigaard N-U, Bryant D (2007) Bacteriochlorophyllide c C-82 and C-121 methyltransferases are essential for adaptation to low light in Chlorobaculum tepidum. J Bacteriol 189(17):6176–6184
Graczyk A, Żurek JM, Paterson MJ (2014) On the linear and non-linear electronic spectroscopy of chlorophylls: a computational study. Photochem Photobiol Sci 13:103–111
Gülen D (2006) Significance of the excitonic intensity borrowing in the J-/H-aggregates of bacteriochlorophylls/chlorophylls. Photosynth Res 87:205–214
Gunther L, Jendrny M, Bloemsma E, Tank M, Oostergetel G, Bryant D, Knoester J, Köhler J (2016) Structure of light-harvesting aggregates in individual chlorosomes. J Phys Chem B 120:5367–5376
Hartigan N, Tharia H, Sweeney F, Lawless A, Papiz M (2002) The 7.5-A electron density and spectroscopic properties of a novel low-light B800 LH2 from Rhodopseudomonas palustris. Biophys J 82:963–977
Holzwarth AR, Schaffner K (1994) On the structure of bacteriochlorophyll molecular aggregates in the chlorosomes of green bacteria. A molecular modelling study. Photosynth Res 41:225–233
Jendrny M, Aartsma T, Kӧhler J (2014) Insights into the excitonic states of individual chlorosomes from Chlorobaculum tepidum. Biophys J 106:1921–1927
Krasnovsky A, Bystrova M (1980) Self-assembly of chlorophyll aggregated structures. BioSystems 12:181–194
Lin S, Van Amerongen H, Struve W (1991) Ultrafast pump-probe spectroscopy of bacteriochlorophyll c antennae in bacteriochlorophyll a-containing chlorosomes from the green photosynthetic bacterium Chloroflexus aurantiacus. Biochim Biophys Acta 1060:13–22
Linnanto J, Korppi-Tommola J (2008) Investigation on chlorosomal antenna geometries: tube, lamella and spiral-type self-aggregates. Photosynth Res 96:227–245
Linnanto J, Korppi-Tommola J (2013) Exciton description of chlorosome to baseplate excitation energy transfer in filamentous anoxygenic phototrophs and green sulfur bacteria. J Phys Chem B 117:11144–11161
Ma Y-Z, Cox R, Gillbro T, Miller M (1996) Bacteriochlorophyll organization and energy transfer kinetics in chlorosomes from Chloroflexus aurantiacus depend on the light regime during growth. Photosynth Res 47:157–165
Martiskainen J, Linnanto J, Kananavičius R, Lehtovuori V, Korppi-Tommola J (2009) Excitation energy transfer in isolated chlorosomes from Chloroflexus aurantiacus. Chem Phys Lett 477:216–220
Martiskainen J, Linnanto J, Aumanen V, Myllyperkio P, Korppi-Tommola J, (2012) Excitation energy transfer in isolated chlorosomes from Chlorobaculum tepidum and Prosthecochloris aestuarii. Photochem Photobiol 88(3):675–683
Mauring K, Novoderezhkin V, Taisova A, Fetisova Z (1999) Exciton levels structure of antenna bacteriochlorophyll c aggregates in the green bacterium Chloroflexus aurantiacus as probed by 1.8–293 K fluorescence spectroscopy. FEBS Lett 456:239–242
Mimuro M, Hirota M, Nishimura Y, Moriyama T, Yamazaki I, Shimada K, Matsuura K (1994) Molecular organization of bacteriochlorophyll in chlorosomes of the green photosynthetic bacterium Chloroflexus aurantiacus: studies of fluorescence depolarization accompanied by energy transfer process. Photosynth Res 41:181–191
Mirkovic T, Ostroumov E, Anna J, van Grondelle R, Govindjee SG (2017) Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem Rev 117(2):249–293
Montaňo G, Wu H, Lin S, Brune D, Blankenship R (2003) Isolation and characterization of the B798 light-harvesting baseplate from the chlorosomes of Chloroflexus aurantiacus. Biochemistry 42:10246–10251
Novoderezhkin V, Taisova A, Fetisova Z, Blankenship R, Savikhin S, Buck D, Struve W (1998) Energy transfers in the B808–866 antenna from the green bacterium Chloroflexus aurantiacus. Biophys J 74:2069–2075
Novoderezhkin V, Taisova A, Fetisova Z (2001) Unit building block of the oligomeric chlorosomal antenna of the green photosynthetic bacterium Chloroflexus aurantiacus: modeling of nonlinear optical spectra. Chem Phys Lett 335:234–240
Oelze J (1992) Light and oxygen regulation of the synthesis of bacteriochlorophyll a and bacteriochlorophyll c in Chloroflexus aurantiacus. J Bacteriol 174:5021–5026
Oelze J, Golecki J (1995) Membranes and chlorosomes of green bacteria: structure, composition and development. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, pp 259–278
Olson JM (1980) Chlorophyll organization in green photosynthetic bacteria. Biochim Biophys Acta 594:33–51
Olson J (1998) Chlorophyll organization and function in green photosynthetic bacteria. Photochem Photobiol 67:61–75
Oostergetel G, van Amerongen H, Boekema E (2010) The chlorosome: a prototype for efficient light harvesting in photosynthesis. Photosynth Res 104(2–3):245–255
Orf G, Blankenship R (2013) Chlorosome antenna complexes from green photosynthetic bacteria. Photosynth Res 116:315–331
Pandit A, de Groot H (2012) Solid-state NMR applied to photosynthetic light-harvesting complexes. Photosynth Res 111:219–226
Pierson B, Castenholz R (1974) Pigments and growth in Chloroflexus aurantiacus, a phototrophic filamentous bacterium. Arch Microbiol 100:283–305
Pierson B, Castenholz R (1992) The family Chloroflexaceae. In: Balows A, Trüper H, Dworkin M, Harder W, Schleifer K (eds) The prokaryotes, vol 4, 2nd edn. Springer, Heidelberg, pp 3754–3774
Pierson B, Castenholz R (1995) Taxonomy and physiology of filamentous anoxygenic phototrophs. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, pp 31–47
Prokhorenko VI, Steensgaard DB, Holzwarth AR (2000) Exciton dynamics in the chlorosomal antennae of the green bacteria Chloroflexus aurantiacus and Chlorobium tepidum. Biophys J 79:2105–2120
Prokhorenko VI, Steensgaard DB, Holzwarth AR (2003) Exciton theory for supramolecular chlorosomal aggregates: 1. Aggregate size dependence of the linear spectra. Biophys J 85:3173–3186
Pšenčik J, Ikonen T, Laurinmäki P, Merckel M, Butcher S, Serimaa R, Tuma R (2004) Lamellar organization of pigments in chlorosomes, the light harvesting system of green bacteria. Biophys J 87:1165–1172
Pšenčik J, Torkkeli M, Zupčanová A, Vácha F, Serimaa R, Tuma R (2010) The lamellar spacing in self-assembling bacteriochlorophyll aggregates is proportional to the length of the esterifying alcohol. Photosynth Res 104:211–219
Pšenčik J, Arellano J, Collins A, Laurinmäki P, Torkkeli M, Lӧflund B, Serimaa R, Blankenship R, Tuma R, Butcher S (2013) Structural and functional roles of carotenoids in chlorosomes. J Bacteriol 195:1727–1734
Rich A, Tinoco I Jr (1960) The effect of chain length upon hypochromism in nucleic acids and polynucleotides. J Am Chem Soc 82:6409–6410
Saga Y, Tamiaki H (2006) Transmission electron microscopic study on supramolecular nanostructures of bacteriochlorophyll self-aggregates in chlorosomes of green photosynthetic bacteria. J Biosc Bioeng 102:18–23
Savikhin S, Zhu Y, Lin S, Blankenship RE, Struve WS (1994) Femtosecond spectroscopy of chlorosome antennas from the green photosynthetic bacterium Chloroflexus aurantiacus. J Phys Chem 98:10322–10334
Savikhin S, Buck D, Struve W, Blankenship R, Taisova A, Novoderezhkin V, Fetisova Z (1998) Exciton delocalization in the bacteriochlorophyll c antenna of the green bacterium Chloroflexus aurantiacus as revealed by ultrafast pump-probe spectroscopy. FEBS Lett 430:323–326
Sawaya N, Huh J, Fujita T, Saikin S, Aspuru-Guzik A (2015) Fast delocalization leads to robust long-range excitonic transfer in a large quantum chlorosome model. Nano Lett 15:1722–1729
Scherz A, Parson WW (1984a) Oligomers of bacteriochlophyll and bacteriophyophytin with spectroscopic properties resembling those found in photosynthetic bacteria. Biochim Biophys Acta 766:653–665
Scherz A, Parson WW (1984b) Exciton interactions in dimers of bacteriochlorophyll and related molecules. Biochim Biophys Acta 766:666–678
Schmidt K, Maarzahl M, Mayer F (1980) Development and pigmentation of chlorosomes in Chloroflexus aurantiacus Ok-70-fl. Arch Microbiol 127:87–97
Scholes GD, Fleming GR, Alexandra Olaya-Castro A, van Grondelle R (2011) Lessons from nature about solar light harvesting. Nat Chem 3:763–774
Shibata Y, Saga Y, Tamiaki H, Itoh S (2006) Low temperature fluorescence from single chlorosomes, photosynthetic antenna complexes of green filamentous and sulfur bacteria. Biophys J 91:3787–3796
Shibata Y, Saga Y, Tamiaki H, Itoh S (2007) Polarized fluorescence of aggregated bacteriochlorophyll c and baseplate bacteriochlorophyll a in single chlorosomes isolated from Chloroflexus aurantiacus. Biochemistry 46:7062–7068
Shibata Y, Tateishi S, Nakabayashi S, Itoh S, Tamiaki H (2010) Intensity borrowing via excitonic couplings among Soret and Qy transitions of bacteriochlorophylls in the pigment aggregates of chlorosomes, the light-harvesting antennae of green sulfur bacteria. Biochemistry 49:7504–7515
Smith K, Kehres L, Fajer J (1983) Aggregation of bacteriochlorophylls c, d or e. Models for the antenna chlorophylls of green and brown photosynthetic bacteria. J Am Chem Soc 105:1387–1389
Sprague S, Staehelin L, DiBartolomeis M, Fuller R (1981) Isolation and development of chlorosomes in the green bacterium Chloroflexus aurantiacus. J Bacteriol 147:1021–1031
Staehelin L, Golecki J, Fuller R, Drews G (1978) Visualization of the supramolecular architecture of chlorosomes (Chlorobium type vesicles) in freeze-fractured cells of Chloroflexus aurantiacus. Arch Microbiol 119:269–277
Taisova A, Gulen D, Iseri E, Drachev V, Cherenkova T, Fetisova Z (2001) Antenna-size dependent hyperchromism of the Qy absorption band of chlorosomal oligomeric bacteriochlorophyll (BChl) c antennae of green bacteria. Photosynth Res 69:9
Taisova A, Keppen O, Lukashev E, Arutyunyan A, Fetisova Z (2002) Study of the chlorosomal antenna of the green mesophilic filamentous bacterium Oscillochloris trichoides. Photosynth Res 74:73–85
Taisova A, Keppen O, Novikov A, Naumova M, Fetisova Z (2006) Some factors controlling the biosynthesis of chlorosome antenna bacteriochlorophylls in green filamentous anoxygenic phototrophic bacteria of the family Oscillochloridaceae. Microbiology 75(2):129–135
Tamiaki H (1996) Supramolecular structure in extramembraneous antennae of green photosynthetic bacteria. Coord Chem Rev 148:183–197
Tamiaki H, Amakawa M, Holzwarth AR, Schaffner K (2002) Aggregation of synthetic metallochlorins in hexane. A model of chlorosomal bacteriochlorophyll self-assemblies in green bacteria. Photosynth Res 71:59–67
Tamiaki H, Shibata R, Mizoguchi T (2007) The 17-propionate function of (bacterio)chlorophylls: biological implication of their long esterifying chains in photosynthetic systems. Photochem Photobiol 83:152–162
Tinoco I (1960) Hypochromism in polynucleotides. J Am Chem Soc 82:4785–4790
Tinoco I Jr (1962) Theoretical Aspects of Optical Activity. Part Two: Polymers Advances in Chemical Physics, vol 4, pp 113–160. Interscience Publishers, New York.
Umetsu M, Wang ZY, Kobayashi M, Nozawa T (1999) Interaction of photosynthetic pigments with various organic solvents: magnetic circular dichroism approach and application to chlorosomes. Biochim Biophys Acta 1410:19–31
Van Dorssen R, Amesz J (1988) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus. III. Energy transfer in whole cells. Photosynth Res 15:177–189
Van Amerongen H, Vasmel H, van Grondelle R (1988) Linear dichroism of chlorosomes from Chloroflexus aurantiacus in compressed gels and electric fields. Biophys J 54:65–76
Van Dorssen RJ, Vasmel H, Amesz J (1986) Pigment organization and energy transfer in the green photosynthetic bacterium Chloroflexus aurantiacus II. The chlorosome. Photosynth Res 9:33–45
Yakovlev A, Novoderezhkin V, Taisova A, Fetisova Z (2002a) Exciton dynamics in the chlorosomal antenna of the green bacterium Chloroflexus aurantiacus: experimental and theoretical studies of femtosecond pump-probe spectra. Photosynth Res 71:19–32
Yakovlev A, Taisova A, Fetisova Z (2002b) Light control over the size of an antenna unit building block as an effecient strategy for light harvesting in photosynthesis. FEBS Lett 512:129–132
Yakovlev A, Taisova A, Arutyunyan A, Shuvalov V, Fetisova Z (2017) Variability of aggregation extent of light-harvesting pigments in peripheral antenna of Chloroflexus aurantiacus. Photosynth Res 133:343–356
Yakovlev AG, Taisova AS, Shuvalov VA, Fetisova ZG (2018) Estimation of the bacteriochlorophyll c oligomerisation extent in Chloroflexus aurantiacus chlorosomes by very low-frequency vibrations of the pigment molecules: a new approach. Biophys Chem 240:1–8
Acknowledgements
The authors are very grateful to Prof. Dr. Demet Gülen for fruitful cooperation and assistance in theoretical modeling. This work was supported in part by the Russian Foundation for Basic Research (Grants 18-04-00105a, 14-04-00295a).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yakovlev, A.G., Taisova, A.S. & Fetisova, Z.G. Q-band hyperchromism and B-band hypochromism of bacteriochlorophyll c as a tool for investigation of the oligomeric structure of chlorosomes of the green photosynthetic bacterium Chloroflexus aurantiacus. Photosynth Res 146, 95–108 (2020). https://doi.org/10.1007/s11120-019-00707-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00707-9