Abstract
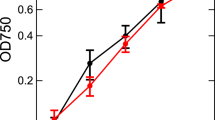
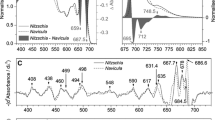
Controlling excitation energy flow is a fundamental ability of photosynthetic organisms to keep a better performance of photosynthesis. Among the organisms, diatoms have unique light-harvesting complexes, fucoxanthin chlorophyll (Chl) a/c-binding proteins. We have recently investigated light-adaptation mechanisms of a marine centric diatom, Chaetoceros gracilis, by spectroscopic techniques. However, it remains unclear how pennate diatoms regulate excitation energy under different growth light conditions. Here, we studied light-adaptation mechanisms in a marine pennate diatom Phaeodactylum tricornutum grown at 30 µmol photons m−2 s−1 and further incubated for 24 h either in the dark, or at 30 or 300 µmol photons m−2 s−1 light intensity, by time-resolved fluorescence (TRF) spectroscopy. The high-light incubated cells showed no detectable oxygen-evolving activity of photosystem II, indicating the occurrence of a severe photodamage. The photodamaged cells showed alterations of steady-state absorption and fluorescence spectra and TRF spectra compared with the dark and low-light adapted cells. In particular, excitation-energy quenching is significantly accelerated in the photodamaged cells as shown by mean lifetime analysis of the Chl fluorescence. These spectral changes by the high-light treatment may result from arrangements of pigment–protein complexes to maintain the photosynthetic performance under excess light illumination. These growth-light dependent spectral properties in P. tricornutum are largely different from those in C. gracilis, thus providing insights into the different light-adaptation mechanisms between the pennate and centric diatoms.





Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- DI:
-
Dark incubated
- FCP:
-
Fucoxanthin chlorophyll a/c-binding protein
- FDA:
-
Fluorescence decay-associated
- HI:
-
High-light incubated
- LI:
-
Low-light incubated
- OD750:
-
Optical density at 750 nm
- PPFD:
-
Photosynthetic photon flux density
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- RC:
-
Reaction center
- TRF:
-
Time-resolved fluorescence
References
Ahn TK, Avenson TJ, Ballottari M, Cheng Y-C, Niyogi KK, Bassi R, Fleming GR (2008) Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320:794–797
Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A (2012) Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1817:1483–1489
Andrizhiyevskaya EG, Chojnicka A, Bautista JA, Diner BA, van Grondelle R, Dekker JP (2005) Origin of the F685 and F695 fluorescence in Photosystem II. Photosynth Res 84:173–180
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, Brzezinski MA, Chaal BK, Chiovitti A, Davis AK, Demarest MS, Detter JC, Glavina T, Goodstein D, Hadi MZ, Hellsten U, Hildebrand M, Jenkins BD, Jurka J, Kapitonov VV, Kröger N, Lau WWY, Lane TW, Larimer FW, Lippmeier JC, Lucas S, Medina M, Montsant A, Obornik M, Parker MS, Palenik B, Pazour GJ, Richardson PM, Rynearson TA, Saito MA, Schwartz DC, Thamatrakoln K, Valentin K, Vardi A, Wilkerson FP, Rokhsar DS (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306:79–86
Biggins J, Bruce D (1989) Regulation of excitation energy transfer in organisms containing phycobilins. Photosynth Res 20:1–34
Blankenship RE (2014) Molecular mechanisms of photosynthesis, 2nd edn. Wiley-Blackwell, Oxford
Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, Rayko E, Salamov A, Vandepoele K, Beszteri B, Gruber A, Heijde M, Katinka M, Mock T, Valentin K, Verret F, Berges JA, Brownlee C, Cadoret JP, Chiovitti A, Choi CJ, Coesel S, De Martino A, Detter JC, Durkin C, Falciatore A, Fournet J, Haruta M, Huysman MJJ, Jenkins BD, Jiroutova K, Jorgensen RE, Joubert Y, Kaplan A, Kröger N, Kroth PG, La Roche J, Lindquist E, Lommer M, Martin-Jézéquel V, Lopez PJ, Lucas S, Mangogna M, McGinnis K, Medlin LK, Montsant A, Oudot-Le Secq MP, Napoli C, Obornik M, Parker MS, Petit JL, Porcel BM, Poulsen N, Robison M, Rychlewski L, Rynearson TA, Schmutz J, Shapiro H, Siaut M, Stanley M, Sussman MR, Taylor AR, Vardi A, von Dassow P, Vyverman W, Willis A, Wyrwicz LS, Rokhsar DS, Weissenbach J, Armbrust EV, Green BR, Van de Peer Y, Grigoriev IV (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244
Brettel K, Leibl W (2001) Electron transfer in photosystem I. Biochim Biophys Acta 1507:100–114
Caffarri S, Broess K, Croce R, van Amerongen H (2011) Excitation energy transfer and trapping in higher plant photosystem II complexes with different antenna sizes. Biophys J 100:2094–2103
Casazza AP, Szczepaniak M, Müller MG, Zucchelli G, Holzwarth AR (2010) Energy transfer processes in the isolated core antenna complexes CP43 and CP47 of photosystem II. Biochim Biophys Acta 1797:1606–1616
Chukhutsina VU, Büchel C, van Amerongen H (2013) Variations in the first steps of photosynthesis for the diatom Cyclotella meneghiniana grown under different light conditions. Biochim Biophys Acta 1827:10–18
Croce R, van Amerongen H (2013) Light-harvesting in photosystem I. Photosynth Res 116:153–166
Croce R, Zucchelli G, Garlaschi FM, Jennings RC (1998) A thermal broadening study of the antenna chlorophylls in PSI-200, LHCI, and PSI core. Biochemistry 37:17355–17360
Croce R, Dorra D, Holzwarth AR, Jennings RC (2000) Fluorescence decay and spectral evolution in intact photosystem I of higher plants. Biochemistry 39:6341–6348
Depauw FA, Rogato A, Ribera d’Alcalá M, Falciatore A (2012) Exploring the molecular basis of responses to light in marine diatoms. J Exp Bot 63:1575–1591
Diner BA, Rappaport F (2002) Structure, dynamics, and energetics of the primary photochemistry of photosystem II of oxygenic photosynthesis. Annu Rev Plant Biol 53:551–580
Edelman M, Mattoo AK (2008) D1-protein dynamics in photosystem II: the lingering enigma. Photosynth Res 98:609–620
Falkowski PG, Katz ME, Knoll AH, Quigg A, Raven JA, Schofield O, Taylor FJR (2004) The evolution of modern eukaryotic phytoplankton. Science 305:354–360
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Flori S, Jouneau PH, Bailleul B, Gallet B, Estrozi LF, Moriscot C, Bastien O, Eicke S, Schober A, Bártulos CR, Maréchal E, Kroth PG, Petroutsos D, Zeeman S, Breyton C, Schoehn G, Falconet D, Finazzi G (2017) Plastid thylakoid architecture optimizes photosynthesis in diatoms. Nat Commun 8:15885
Gobets B, van Grondelle R (2001) Energy transfer and trapping in photosystem I. Biochim Biophys Acta 1507:80–99
Gobets B, van Stokkum IHM, Rögner M, Kruip J, Schlodder E, Karapetyan NV, Dekker JP, van Grondelle R (2001) Time-resolved fluorescence emission measurements of photosystem I particles of various cyanobacteria: a unified compartmental model. Biophys J 81:407–424
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Green BR, Pichersky E (1994) Hypothesis for the evolution of three-helix Chl a/b and Chl a/c light-harvesting antenna proteins from two-helix and four-helix ancestors. Photosynth Res 39:149–162
Groot M-L, Peterman EJG, van Stokkum IHM, Dekker JP, van Grondelle R (1995) Triplet and fluorescing states of the CP47 antenna complex of photosystem II studied as a function of temperature. Biophys J 68:281–290
Grouneva I, Rokka A, Aro E-M (2011) The thylakoid membrane proteome of two marine diatoms outlines both diatom-specific and species-specific features of the photosynthetic machinery. J Proteome Res 10:5338–5353
Gundermann K, Schmidt M, Weisheit W, Mittag M, Büchel C (2013) Identification of several sub-populations in the pool of light harvesting proteins in the pennate diatom Phaeodactylum tricornutum. Biochim Biophys Acta 1827:303–310
Hamada F, Yokono M, Hirose E, Murakami A, Akimoto S (2012) Excitation energy relaxation in a symbiotic cyanobacterium, Prochloron didemni, occurring in coral-reef ascidians, and in a free-living cyanobacterium, Prochlorothrix hollandica. Biochim Biophys Acta 1817:1992–1997
Hamada F, Murakami A, Akimoto S (2017) Adaptation of divinyl chlorophyll a/b-containing cyanobacterium to different light conditions: three strains of Prochlorococcus marinus. J Phys Chem B 121:9081–9090
Horton P, Ruban A (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56:365–373
Ihalainen JA, van Stokkum IHM, Gibasiewicz K, Germano M, van Grondelle R, Dekker JP (2005) Kinetics of excitation trapping in intact Photosystem I of Chlamydomonas reinhardtii and Arabidopsis thaliana. Biochim Biophys Acta 1706:267–275
Ikeda Y, Komura M, Watanabe M, Minami C, Koike H, Itoh S, Kashino Y, Satoh K (2008) Photosystem I complexes associated with fucoxanthin-chlorophyll-binding proteins from a marine centric diatom, Chaetoceros gracilis. Biochim Biophys Acta 1777:351–361
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Kashino Y, Kudoh S (2003) Concerted response of xanthophyll-cycle pigments in a marine diatom, Chaetoceros gracilis, to shifts in light condition. Phycol Res 51:168–172
Lavaud J, Lepetit B (2013) An explanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms. Biochim Biophys Acta 1827:294–302
Lepetit B, Volke D, Szabó M, Hoffmann R, Garab G, Wilhelm C, Goss R (2007) Spectroscopic and molecular characterization of the oligomeric antenna of the diatom Phaeodactylum tricornutum. Biochemistry 46:9813–9822
Lepetit B, Volke D, Gilbert M, Wilhelm C, Goss R (2010) Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms. Plant Physiol 154:1905–1920
Lepetit B, Gélin G, Lepetit M, Sturm S, Vugrinec S, Rogato A, Kroth PG, Falciatore A, Lavaud J (2017) The diatom Phaeodactylum tricornutum adjusts nonphotochemical fluorescence quenching capacity in response to dynamic light via fine-tuned Lhcx and xanthophyll cycle pigment synthesis. New Phytol 214:205–218
Lohr M, Wilhelm C (1999) Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc Natl Acad Sci U S A 96:8784–8789
Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45:633–662
Ma Y-Z, Holt NE, Li X-P, Niyogi KK, Fleming GR (2003) Evidence for direct carotenoid involvement in the regulation of photosynthetic light harvesting. Proc Natl Acad Sci U S A 100:4377–4382
MacIntyre HL, Kana TM, Geider RJ (2000) The effect of water motion on short-term rates of photosynthesis by marine phytoplankton. Trends Plant Sci 5:12–17
Miloslavina Y, Grouneva I, Lambrev PH, Lepetit B, Goss R, Wilhelm C, Holzwarth AR (2009) Ultrafast fluorescence study on the location and mechanism of non-photochemical quenching in diatoms. Biochim Biophys Acta 1787:1189–1197
Mimuro M, Akimoto S, Tomo T, Yokono M, Miyashita H, Tsuchiya T (2007) Delayed fluorescence observed in the nanosecond time region at 77 K originates directly from the photosystem II reaction center. Biochim Biophys Acta 1767:327–334
Mimuro M, Yokono M, Akimoto S (2010) Variations in photosystem I properties in the primordial cyanobacterium Gloeobacter violaceus PCC 7421. Photochem Photobiol 86:62–69
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murata N, Allakhverdiev SI, Nishiyama Y (2012) The mechanism of photoinhibition in vivo: re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim Biophys Acta 1817:1127–1133
Nagao R, Ishii A, Tada O, Suzuki T, Dohmae N, Okumura A, Iwai M, Takahashi T, Kashino Y, Enami I (2007) Isolation and characterization of oxygen-evolving thylakoid membranes and Photosystem II particles from a marine diatom Chaetoceros gracilis. Biochim Biophys Acta 1767:1353–1362
Nagao R, Tomo T, Noguchi E, Nakajima S, Suzuki T, Okumura A, Kashino Y, Mimuro M, Ikeuchi M, Enami I (2010) Purification and characterization of a stable oxygen-evolving Photosystem II complex from a marine centric diatom, Chaetoceros gracilis. Biochim Biophys Acta 1797:160–166
Nagao R, Takahashi S, Suzuki T, Dohmae N, Nakazato K, Tomo T (2013a) Comparison of oligomeric states and polypeptide compositions of fucoxanthin chlorophyll a/c-binding protein complexes among various diatom species. Photosynth Res 117:281–288
Nagao R, Yokono M, Akimoto S, Tomo T (2013b) High excitation energy quenching in fucoxanthin chlorophyll a/c-binding protein complexes from the diatom Chaetoceros gracilis. J Phys Chem B 117:6888–6895
Nagao R, Yokono M, Teshigahara A, Akimoto S, Tomo T (2014a) Light-harvesting ability of the fucoxanthin chlorophyll a/c-binding protein associated with photosystem II from the diatom Chaetoceros gracilis as revealed by picosecond time-resolved fluorescence spectroscopy. J Phys Chem B 118:5093–5100
Nagao R, Yokono M, Tomo T, Akimoto S (2014b) Control mechanism of excitation energy transfer in a complex consisting of photosystem II and fucoxanthin chlorophyll a/c-binding protein. J Phys Chem Lett 5:2983–2987
Nagao R, Tomo T, Narikawa R, Enami I, Ikeuchi M (2016) Conversion of photosystem II dimer to monomers during photoinhibition is tightly coupled with decrease in oxygen-evolving activity in the diatom Chaetoceros gracilis. Photosynth Res 130:83–91
Nagao R, Ueno Y, Akita F, Suzuki T, Dohmae N, Akimoto S, Shen J-R (2018a) Biochemical characterization of photosystem I complexes having different subunit compositions of fucoxanthin chlorophyll a/c-binding proteins in the diatom Chaetoceros gracilis. Photosynth Res https://doi.org/10.1007/s11120-018-0576-y
Nagao R, Ueno Y, Yokono M, Shen J-R, Akimoto S (2018b) Alterations of pigment composition and their interactions in response to different light conditions in the diatom Chaetoceros gracilis probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1859:524–530
Nagao R, Yokono M, Ueno Y, Shen J-R, Akimoto S (2019) Low-energy chlorophylls in fucoxanthin chlorophyll a/c-binding protein conduct excitation energy transfer to photosystem I in diatoms. J Phys Chem B 123:66–70
Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43:11321–11330
Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–578
Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Shibata Y, Nishi S, Kawakami K, Shen J-R, Renger T (2013) Photosystem II does not possess a simple excitation energy funnel: time-resolved fluorescence spectroscopy meets theory. J Am Chem Soc 135:6903–6914
Slavov C, Ballottari M, Morosinotto T, Bassi R, Holzwarth AR (2008) Trap-limited charge separation kinetics in higher plant photosystem I complexes. Biophys J 94:3601–3612
Taddei L, Chukhutsina VU, Lepetit B, Stella GR, Bassi R, van Amerongen H, Bouly J-P, Jaubert M, Finazzi G, Falciatore A (2018) Dynamic changes between two LHCX-related energy quenching sites control diatom photoacclimation. Plant Physiol 177:953–965
Tyystjärvi E (2008) Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 252:361–376
van der Weij-de Wit CD, Ihalainen JA, van Grondelle R, Dekker JP (2007) Excitation energy transfer in native and unstacked thylakoid membranes studied by low temperature and ultrafast fluorescence spectroscopy. Photosynth Res 93:173–182
van Grondelle R, Dekker JP, Gillbro T, Sundstrom V (1994) Energy transfer and trapping in photosynthesis. Biochim Biophys Acta 1187:1–65
Veith T, Büchel C (2007) The monomeric photosystem I-complex of the diatom Phaeodactylum tricornutum binds specific fucoxanthin chlorophyll proteins (FCPs) as light-harvesting complexes. Biochim Biophys Acta 1767:1428–1435
Wang W, Yu L-J, Xu C, Tomizaki T, Zhao S, Umena Y, Chen X, Qin X, Xin Y, Suga M, Han G, Kuang T, Shen J-R (2019) Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science 363:eaav0365
Wlodarczyk LM, Dinc E, Croce R, Dekker JP (2016) Excitation energy transfer in Chlamydomonas reinhardtii deficient in the PSI core or the PSII core under conditions mimicking state transitions. Biochim Biophys Acta 1857:625–633
Yokono M, Akimoto S, Koyama K, Tsuchiya T, Mimuro M (2008a) Energy transfer processes in Gloeobacter violaceus PCC 7421 that possesses phycobilisomes with a unique morphology. Biochim Biophys Acta 1777:55–65
Yokono M, Akimoto S, Tanaka A (2008b) Seasonal changes of excitation energy transfer and thylakoid stacking in the evergreen tree Taxus cuspidata: how does it divert excess energy from photosynthetic reaction center? Biochim Biophys Acta 1777:379–387
Yokono M, Murakami A, Akimoto S (2011) Excitation energy transfer between photosystem II and photosystem I in red algae: larger amounts of phycobilisome enhance spillover. Biochim Biophys Acta 1807:847–853
Yokono M, Tomo T, Nagao R, Ito H, Tanaka A, Akimoto S (2012) Alterations in photosynthetic pigments and amino acid composition of D1 protein change energy distribution in photosystem II. Biochim Biophys Acta 1817:754–759
Yokono M, Nagao R, Tomo T, Akimoto S (2015a) Regulation of excitation energy transfer in diatom PSII dimer: how does it change the destination of excitation energy? Biochim Biophys Acta 1847:1274–1282
Yokono M, Takabayashi A, Akimoto S, Tanaka A (2015b) A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun 6:6675
Yokono M, Takabayashi A, Kishimoto J, Fujita T, Iwai M, Murakami A, Akimoto S, Tanaka A (2019) The PSI–PSII megacomplex in green plants. Plant Cell Physiol https://doi.org/10.1093/pcp/pcz026
Acknowledgements
This work was supported by the Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science JP17K07442 (to R. N.), JP17H06433 (to J.-R. S.), and JP16H06553 (to S. A.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagao, R., Ueno, Y., Yokono, M. et al. Effects of excess light energy on excitation-energy dynamics in a pennate diatom Phaeodactylum tricornutum. Photosynth Res 141, 355–365 (2019). https://doi.org/10.1007/s11120-019-00639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00639-4