Abstract
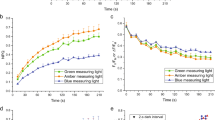
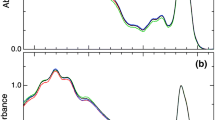
Eustigmatophyte algae represent an interesting model system for the study of the regulation of the excitation energy flow due to their use of violaxanthin both as a major light-harvesting pigment and as the basis of xanthophyll cycle. Fluorescence induction kinetics was studied in an oleaginous marine alga Nannochloropsis oceanica. Nonphotochemical fluorescence quenching was analyzed in detail with respect to the state of the cellular xanthophyll pool. Two components of nonphotochemical fluorescence quenching (NPQ), both dependent on the presence of zeaxanthin, were clearly resolved, denoted as slow and fast NPQ based on kinetics of their formation. The slow component was shown to be in direct proportion to the amount of zeaxanthin, while the fast NPQ component was transiently induced in the presence of membrane potential on subsecond timescales. The applicability of these observations to other eustigmatophyte species is demonstrated by measurements of other representatives of this algal group, both marine and freshwater.









Similar content being viewed by others
Abbreviations
- AL:
-
Actinic illumination
- Ant:
-
Antheraxanthin
- Chl:
-
Chlorophyll
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- DEPS:
-
Xanthophyll pool de-epoxidation state
- NPQ:
-
Nonphotochemical (Chl a fluorescence) quenching
- PSII:
-
Photosystem II
- SP:
-
Saturating pulse
- Vio:
-
Violaxanthin
- Zea:
-
Zeaxanthin
References
Bailleul B, Rogato A, de Martino A, Coesel S, Cardole P, Bowler C, Falciatore A, Finazzi G (2010) An atypical member of the light-harvesting complex stress-related protein family modulates diatom responses to light. Proc Natl Acad Sci USA 107:18214–18219
Berera R, van Stokkum IHM, d’Haene S, Kennis JTM, van Grondelle R, Dekker JP (2009) A mechanism of energy dissipation in Cyanobacteria. Biophys J 96:2261–2267
Bilger W, Schreiber U (1986) Energy-dependent quenching of dark-level chlorophyll fluorescence in intact leaves. Photosynth Res 10:303–308
Bína D, Litvín R, Vácha F (2009) Kinetics of in vivo bacteriochlorophyll fluorescence yield and the state of photosynthetic apparatus of purple bacteria. Photosynth Res 99:115–125
Bína D, Gardian Z, Herbstová M, Kotabová E, Koník P, Litvín R, Prášil O, Tichý J, Vácha F (2014) Novel type of red-shifted chlorophyll a antenna complex from Chromera velia II. Biochemistry and spectroscopy. Biochim Biophys Acta 1837:802–810
Cao S, Zhang X, Xu D, Fan X, Mou S, Wanga Y, Ye N, Wang W (2013) A transthylakoid proton gradient and inhibitors induce a non-photochemical fluorescence quenching in unicellular algae Nannochloropsis sp. FEBS Lett 587:1310–1315
Dittami S, Michel G, Collén J, Boyen C, Tonon T (2010) Chlorophyll-binding proteins revisited—a multigenic family of light-harvesting and stress proteins from a brown algal perspective. Evol Biol 10:365
Dong Y-L, Jiang T, Xia W, Dong H-P, Lu S-H, Cui L (2015) Light harvesting proteins regulate non-photochemical fluorescence quenching in the marine diatom Thalassiosira pseudonana. Algal Res 12:300–307
Fawley KP, Eliáš M, Fawley MW (2014) The diversity and phylogeny of the commercially important algal class Eustigmatophyceae, including the new clade Goniochloridales. J Appl Phycol 26:1773–1782
Fujii R, Kita M, Iinuma Y, Oka N, Takaesu Y, Taira T, Iha M, Cogdell RJ, Hashimoto H (2012) Isolation and purification of the major photosynthetic antenna, fucoxanthin-Chl a/c protein, from cultured discoid germilings of the brown Alga, Cladosiphon okamuranus TOKIDA (Okinawa Mozuku). Photosynth Res 111:157–163
García-Mendoza E, Colombo-Pallotta MF (2007) The giant kelp Macrocystis pyrifera presents a different nonphotochemical quenching control than higher plants. New Phytol 173:526–536
Garcia-Mendoza E, Ocampo-Alvarez H, Govindjee (2011) Photoprotection in the brown alga Macrocystis pyrifera: evolutionary implications. J Photochem Photobiol B 104:377–385
Gentile M-P, Blanch HW (2001) Physiology and xanthophyll cycle activity of Nannochloropsis gaditana. Biotechnol Bioengy 75:1–12
Gilmore AM, Björkman O (1995) Temperature-sensitive coupling and uncoupling of ATP-mediated nonradiative energy dissipation: similarities between chloroplasts and leaves. Planta 197:646–654
Gilmore AM, Yamamoto HY (1993) Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res 35:67–78
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Goss R, Böhme K, Wilhelm C (1998) The xanthophyll cycle of Mantoniella squamata converts violaxanthin into antheraxanthin but not to zeaxanthin: consequences for the mechanism of enhanced non-photochemical energy dissipation. Planta 205:613–621
Goss R, Lepetit B, Wilhelm C (2006) Evidence for a rebinding of antheraxanthin to the light-harvesting complex during the epoxidation reaction of the violaxanthin cycle. J Plant Physiol 163:585–590
Grouneva I, Jakob T, Wilhelm C, Goss R (2008) A new multicomponent NPQ mechanism in the diatom Cyclotella meneghiniana. Plant Cell Physiol 49:1217–1225
Grouneva I, Jakob T, Wilhelm C, Goss R (2009) The regulation of xanthophyll cycle activity and of non-photochemical fluorescence quenching by two alternative electron flows in the diatoms Phaeodactylum tricornutum and Cyclotella meneghiniana. Biochim Biophys Acta 1787:929–938
Guillard RRL, Lorenzen CJ (1972) Yellow-green algae with chlorophyllide c. J Phycol 8:10–14
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Gundermann K, Büchel C (2008) The fluorescence yield of the trimeric fucoxanthin-chlorophyll-protein FCPa in the diatom Cyclotella meneghiniana is dependent on the amount of bound diatoxanthin. Photosynth Res 95:229–235
Herbstová M, Bína D, Koník P, Gardian Z, Vácha F, Litvín R (2015) Molecular basis of chromatic adaptation in pennate diatom Phaeodactylum tricornutum. Biochim Biophys Acta 1847:534–543
Hohmann-Marriott MF, Blankenship RE (2007) Variable fluorescence in green sulfur bacteria. Biochim Biophys Acta 1767:106–113
Jeffrey SW, Mantoura RFC, Wright SW (2005) Phytoplankton pigments in oceanography: guidelines to modern methods, 2nd edn. Monographs on oceanographic methodology, vol 10. UNESCO Publishing, Paris
Keşan G, Litvín R, Bína D, Durchan M, Šlouf V, Polívka T (2016) Efficient light-harvesting using non-carbonyl carotenoids: energy transfer dynamics in the VCP complex from Nannochloropsis oceanica. Biochim Biophys Acta 1857:370–379
Kirilovsky D (2010) The photoactive orange carotenoid protein and photoprotection in cyanobacteria. Adv Exp Med Biol 675:139–159
Konotchick T, Dupont CL, Valas RE, Badger JH, Allen AE (2013) Transcriptomic analysis of metabolic function in the giant kelp, Macrocystis pyrifera, across depth and season. New Phytol 198:398–407
Kotabová E, Kaňa R, Jarešová J, Prášil O (2011) Non-photochemical fluorescence quenching in Chromera velia is enabled by fast violaxanthin de-epoxidation. FEBS Lett 585:1941–1945
Lavaud J, Lepetit B (2013) An explanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms. Biochim Biophys Acta 1827:294–302
Lavaud J, Rousseau B, Etienne A-L (2002) In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett 523:163–166
Lepetit B, Volke D, Gilbert M, Wilhelm C, Goss R (2010) Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms. Plant Physiol 154:1905–1920
Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Nyoigi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Litvín R, Bína D, Herbstová M, Gardian Z (2016) Architecture of the light harvesting apparatus of the eustigmatophyte alga Nannochloropsis oceanica. Photosynth Res. doi:10.1007/s11120-016-0234-1
Lubián LM, Montero O (1998) Excess light-induced violaxanthin cycle activity in Nannochloropsis gaditana (Eustigmatophyceae): effects of exposure time and temperature. Phycologia 37:16–23
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Nilkens M, Eugen Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797:466–475
Ocampo-Alvarez H, García-Mendoza E, Govindjee (2013) Antagonist effect between violaxanthin and de-epoxidated pigments in nonphotochemical quenching induction in the qE deficient brown alga Macrocystis pyrifera. Biochim Biophys Acta 1827:427–437
Owens TG, Gallagher JC, Alberte RS (1987) Photosynthetic light harvesting function of violaxanthin in Nannochloropsis spp. (Eustigmatophyceae). J Phycol 23:79–85
Pan H, Šlapeta J, Carter D, Chen M (2012) Phylogenetic analysis of the light-harvesting system in Chromera velia. Photosynth Res 111:19–28
Reynolds JM, Bruns BU, Fitt WK, Schmidt GW (2008) Enhanced photoprotection pathways in symbiotic dinoflagellates of shallow-water corals and other cnidarians. Proc Natl Acad Sci USA 105:13674–13678
Roháček K, Bertrand M, Moreau B, Jacquette B, Caplat C, Morant-Manceau A, Schoefs B (2014) Relaxation of the non-photochemical chlorophyll fluorescence quenching in diatoms: kinetics, components and mechanisms. Phil Trans R Soc B 369:20130241
Simionato D, Sforza E, Carpinelli EC, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresour Technol 102:6026–6032
Stransky H, Hager A (1970) The carotenoid pattern and the occurrence of the light-induced xanthophyll cycle in various classes of algae. II. Xanthophyceae. Arch Microbiol 71:164–190
Ting CS, Owens TG (1994) The effects of excess irradiance on photosynthesis in the marine diatom Phaeodactylum tricornutum. Plant Physiol 106:763–770
Tsuyama M, Shibata M, Kawazu T, Kobayashi Y (2004) An analysis of the mechanism of the low-wave phenomenon of chlorophyll fluorescence. Photosynth Res 81:67–76
Xyländer M, Hagen C (2002) ‘Low-waves’ in chlorophyll fluorescence kinetics indicate deprivation of bicarbonate. Photosynth Res 72:255–262
Zapata M, Garrido JL, Jeffrey SW (2006) Chlorophyll c pigments: current status. In: Grimm B, Porra RJ, Rüdiger W, Scheer H (eds) Chlorophylls and Bacteriochlorophylls: biochemistry, biophysics, functions and applications. Kluwer, Dordrecht, pp 39–53
Acknowledgments
This work was supported by the Czech Science Foundation grant 14-01377P and by institutional funding RVO:60077344. K.B. was involved in the present work during his participation in a secondary school student scientific project competition. The authors would like to thank Vladimír Špunda for fruitful discussion on the ‘low-wave’ phenomenon. Expert technical assistance of Mr. František Matoušek is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bína, D., Bouda, K. & Litvín, R. A two-component nonphotochemical fluorescence quenching in eustigmatophyte algae. Photosynth Res 131, 65–77 (2017). https://doi.org/10.1007/s11120-016-0299-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0299-x