Abstract
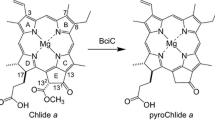
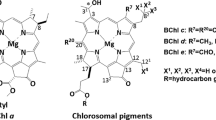
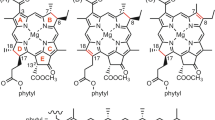
The photosynthetic green sulfur bacterium Chlorobaculum (Cba.) tepidum produces bacteriochlorophyll (BChl) c pigments bearing a chiral 1-hydroxyethyl group at the 3-position, which self-aggregate to construct main light-harvesting antenna complexes, chlorosomes. The secondary alcoholic hydroxy group is requisite for chlorosomal aggregation and biosynthesized by hydrating the 3-vinyl group of their precursors. Using recombinant proteins of Cba. tepidum BchF and BchV, we examined in vitro enzymatic hydration of some 3-vinyl-chlorophyll derivatives. Both the enzymes catalyzed stereoselective hydration of zinc 3-vinyl-8-ethyl-12-methyl-bacteriopheophorbide c or d to the zinc 31 R-bacteriopheophorbide c or d homolog, respectively, with a slight amount of the 31 S-epimric species. A similar R-stereoselectivity was observed in the BchF-hydration of zinc 3-vinyl-8-ethyl- and propyl-12-ethyl-bacteriopheophorbides c, while their BchV-hydration gave a relatively larger amount of the 31 S-epimers. The in vitro stereoselective hydration confirmed the in vivo production of the S-epimeric species by BchV. The enzymatic hydration for the above 8-propylated substrate proceeded more slowly than that for the 8-ethylated, and the 8-isobutylated substrate was no longer hydrated. Based on these results, biosynthetic pathways of BChl c homologs and epimers are proposed.








Similar content being viewed by others
Abbreviations
- BChl:
-
Bacteriochlorophyll
- BPheide:
-
Bacteriopheophorbide
- BPheo:
-
Bacteriopheophytin
- Cba. :
-
Chlorobaculum
- Chl:
-
Chlorophyll
- Chlide:
-
Chlorophyllide
- PChlide:
-
Protochlorophyllide
- Pheide:
-
Pheophorbide
- R[E,E]:
-
(31 R)-8,12-diethyl
- R[E,M]:
-
(31 R)-8-ethyl-12-methyl
- R[P,E]:
-
(31 R)-8-propyl-12-ethyl
- S[I,E]:
-
(31 S)-8-isobutyl-12-ethyl
- S[P,E]:
-
(31 S)-8-propyl-12-ethyl
- THF:
-
Tetrahydrofuran
- 3V:
-
3-Vinyl
References
Blankenship RE (2014) Molecular mechanism of photosynthesis, 2nd edn. Wiley Blackwell, West Sussex, pp 1–9
Blankenship RE, Matsuura K (2003) Antenna complexes from green photosynthetic bacteria. In: Green BR, Parson WW (eds) Light-harvesting antennas in photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 195–217
Bryant DA, Liu Z (2013) Green bacteria: insights into green bacterial evolution through genomic analyses. Adv Bot Res 66:99–150
Bryant DA, Costas AMG, Maresca JA, Chew AGM, Klatt CG, Bateson MM, Tallon LJ, Hostetler J, Nelson WC, Heidelberg JF, Ward DM (2007) Candidatus Chloracidobacterium thermophilum: an aerobic phototrophic acidobacterium. Science 317:523–526
Chew AGM, Bryant DA (2007) Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annu Rev Microbiol 61:113–129
Chew AGM, Frigaard N-U, Bryant DA (2004) Identification of BchV, a C-31 hydratase specific for hypermethylated bacteriochlorophyll c in Chlorobaculum tepidum. In: van der Est A, Bruce D (eds) Photosynthesis: fundamental aspects to global perspectives research. Allen Press, Lawrence, pp 875–877
Chew AGM, Frigaard N-U, Bryant DA (2007) Bacteriochlorophyllide c C-82 and C-121 methyltransferases are essential for adaptation to low light in Chlorobaculum tepidum. J Bacteriol 189:6176–6184
Cogdell RJ, Gall A, Köhler J (2006) The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes. Q Rev Biophys 39:227–324
Fages F, Griebenow N, Griebenow K, Holzwarth AR, Schaffner K (1990) Characterization of light-harvesting pigments of Chloroflexus aurantiacus. Two new chlorophylls: oleyl (octadec-9-enyl) and cetyl (hexadecanyl) bacteriochlorophyllides-c. J Chem Soc Perkin Trans 1:2791–2797
Fajer J (2004) Chlorophyll chemistry before and after crystals of photosynthetic reaction centers. Photosynth Res 80:165–172
Frigaard N-U, Voigt GD, Bryant DA (2002) Chlorobium tepidum mutant lacking bacteriochlorophyll c made by inactivation of the bchK gene, encoding bacteriochlorophyll c synthase. J Bacteriol 184:3368–3376
Ganapathy S, Oostergetel GT, Wawrzyniak PK, Reus M, Chew AGM, Buda F, Boekema EJ, Bryant DA, Holzwarth AR, de Groot HJ (2009) Alternating syn-anti bacteriochlorophylls form concentric helical nanotubes in chlorosomes. Proc Natl Acad Sci USA 106:8525–8530
Harada J, Saga Y, Yaeda Y, Oh-oka H, Tamiaki H (2005) In vitro activity of C-20 methyltransferase, BchU, involved in bacteriochlorophyll c biosynthesis pathway in green sulfur bacteria. FEBS Lett 579:1983–1987
Harada J, Mizoguchi T, Tsukatani Y, Noguchi M, Tamiaki H (2012) A seventh bacterial chlorophyll driving a large light-harvesting antenna. Sci Rep 2:671. doi:10.1038/srep00671
Harada J, Mizoguchi T, Nomura K, Tamiaki H (2014) Isolation and structural determination of C8-vinyl-bacteriochlorophyll d from the bciA and bchU double mutant of the green sulfur bacterium Chlorobaculum tepidum. Photosynth Res 121:13–23
Harada J, Teramura M, Mizoguchi T, Tsukatani Y, Yamamoto K, Tamiaki H (2015) Stereochemical conversion of the 3-vinyl group to 3-(1-hydroxyethyl) group in bacteriochlorophyll c by the hydratases BchF and BchV: adaptation of green sulfur bacteria to limited-light environments. Mol Microbiol 98:1184–1198
Hartzler DA, Niedzwiedzki DM, Bryant DA, Blankenship RE, Pushkar Y, Savikhin S (2014) Triplet excited state energies and phosphorescence spectra of (bacterio)chlorophylls. J Phys Chem B 118:7221–7232
Iriyama K, Ogura N, Takamiya A (1974) A simple method for extraction and partial purification of chlorophyll from plant material, using dioxane. J Biochem 76:901–904
Kobayashi M, Akutsu S, Fujinuma D, Komatsu H, Kato Y, Kuroiwa Y, Watanabe T, Ohnishi-Kameyama M, Ono H, Ohkubo S, Miyashita H (2013) Physicochemical properties of chlorophylls in oxygenic photosynthesis—succession of co-factors from anoxygenic to oxygenic photosynthesis. In: Dubinsky Z (ed) Photosynthesis. InTech, pp 47–90
Lange C, Kiesel S, Peter S, Virus S, Scheer H, Jahn D, Moser J (2015) Broadened substrate specificity of 3-hydroxyethyl bacteriochlorophyll a dehydrogenase (BchC) indicates a new route for the biosynthesis of bacteriochlorophyll a. J Biol Chem 290:19697–19709
Liu Z, Bryant DA (2011) Identification of gene essential for the first committed step in the biosynthesis of bacteriochlorophyll c. J Biol Chem 286:22393–22402
Maresca JA, Chew AGM, Ponsatí MR, Frigaard N-U, Ormerod JG, Bryant DA (2004) The bchU gene of Chlorobium tepidum encodes the C-20 methyltransferase in bacteriochlorophyll c biosynthesis. J Bacteriol 186:2558–2566
Miyatake T, Tamiaki H (2005) Self-aggregates of bacteriochlorophylls-c, d and e in a light-harvesting antenna system of green photosynthetic bacteria: effect of stereochemistry at the chiral 3-(1-hydroxyethyl) group on the supramolecular arrangement of chlorophyllous pigments. J Photochem Photobiol C 6:89–107
Mizoguchi T, Nagai C, Kunieda M, Kimura Y, Okamura A, Tamiaki H (2009) Stereochemical determination of the unique acrylate moiety at the 17-position in chlorophylls-c from a diatom Chaetoceros calcitrans and its effect upon electronic absorption properties. Org Biomol Chem 7:2120–2126
Mizoguchi T, Harada J, Tsukatani Y, Tamiaki H (2014) Isolation and characterization of a new bacteriochlorophyll-c bearing a neopentyl substituent at the 8-position from the bciD-deletion mutant of the brown-colored green sulfur bacterium Chlorobaculum limnaeum. Photosynth Res 121:3–12
Mizoguchi T, Harada J, Yamamoto K, Tamiaki H (2015) Inactivation of bciD and bchU genes in the green sulfur bacterium Chlorobaculum limnaeum and alteration of photosynthetic pigments in the resultant mutants. J Photochem Photobiol A 313:52–59
Muraki N, Nomata J, Ebata K, Mizoguchi T, Shiba T, Tamiaki H, Kurisu G, Fujita Y (2010) X-ray crystal structure of the light-independent protochlorophyllide reductase. Nature 465:110–114
Niedzwiedzki DM, Blankenship RE (2010) Singlet and triplet excited state properties of natural chlorophylls and bacteriochlorophylls. Photosynth Res 106:227–238
Nomata J, Mizoguchi T, Tamiaki H, Fujita Y (2006) A second nitrogenase-like enzyme for bacteriochlorophyll biosynthesis: reconstitution of chlorophyllide a reductase with purified X-protein (BchX) and YZ-protein (BchY-BchZ) from Rhodobacter capsulatus. J Biol Chem 281:15021–15028
Oba T, Tamiaki H (1991) Why do chlorosomal chlorophylls lack the C132-methoxycarbonyl moiety? an in vitro model study. Photosynth Res 61:23–31
Olson JM (1998) Chlorophyll organization and function in green photosynthetic bacteria. Photochem Photobiol 67:61–75
Orf GS, Blankenship RE (2013) Chlorosome antenna complexes from green photosynthetic bacteria. Photosynth Res 116:315–331
Orf GS, Tank M, Vogl K, Niedzwiedzki DM, Bryant DA, Blankenship RE (2013) Spectroscopic insights into the decreased efficiency of chlorosomes containing bacteriochlorophyll f. Biochim Biophys Acta 1827:493–501
Ryan AA, Senge MO (2015) How green is green chemistry? Chlorophyll as a bioresource from biorefineries and their commercial potential in medicine and photovoltaics. Photochem Photobiol Sci 14:638–660
Saga Y, Hirota K, Harada J, Tamiaki H (2015) In vitro enzymatic activities of bacteriochlorophyll a synthase derived from the green sulfur photosynthetic bacterium Chlorobaculum tepidum. Biochemistry 54:4998–5005
Tamiaki H (1996) Supramolecular structure in extramembraneous antennae of green photosynthetic bacteria. Coord Chem Rev 148:183–197
Tamiaki H, Kunieda M (2011) Photochemistry of chlorophylls and their synthetic analogs. In: Kadish KM, Smith KM, Guilard R (eds) Handbook of porphyrin science, vol 11. World Scientific Publishing, Singapore, pp 223–290
Tamiaki H, Amakawa M, Shimono Y, Tanikaga R, Holzwarth AR, Schaffner K (1996) Synthetic zinc and magnesium chlorin aggregates as models for supramolecular antenna complexes in chlorosomes of green photosynthetic bacteria. Photochem Photobiol 63:92–99
Tamiaki H, Takeuchi S, Tsudzuki S, Miyatake T, Tanikaga R (1998) Self-aggregation of synthetic zinc chlorins with a chiral 1-hydroxyethyl group as a model for in vivo epimeric bacteriochlorophyll-c and d aggregates. Tetrahedron 54:6699–6718
Tamiaki H, Shibata R, Mizoguchi T (2007) The 17-propionate function of (bacterio)chlorophylls: biological implication of their long esterifying chains in photosynthetic system. Photochem Photobiol 83:152–162
Tamiaki H, Machida S, Mizutani K (2012) Modification of 3-substituents in (bacterio)chlorophyll derivatives to prepare 3-ethylated, methylated, and unsubstituted (nickel) pyropheophorbides and their optical properties. J Org Chem 77:4751–4758
Tamiaki H, Teramura M, Tsukatani Y (2016) Reduction processes in biosynthesis of chlorophyll molecules: chemical implication of enzymatically regio- and stereoselective hydrogenations in the late stages of their biosynthetic pathway. Bull Chem Soc Jpn 89:doi:10.1246/bcsj.20150307
Tsukatani Y, Yamamoto H, Harada J, Yoshitomi T, Nomata J, Kasahara M, Mizoguchi T, Fujita Y, Tamiaki H (2013) An unexpectedly branched biosynthetic pathway for bacteriochlorophyll b capable of absorbing near-infrared light. Sci Rep 3:1217. doi:10.1038/srep01217
Wakao N, Yokoi N, Isoyama N, Hiraishi A, Shimada K, Kobayashi M, Kise H, Iwaki M, Itoh S, Takaichi S, Sakurai Y (1996) Discovery of natural photosynthesis using Zn-containing bacteriochlorophyll in an aerobic bacterium Acidiphilium rubrum. Plant Cell Physiol 37:889–893
Xu M, Kinoshita Y, Tamiaki H (2014) Synthesis of chlorophyll-f analogs possessing the 2-formyl group by modifying chlorophyll-a. Bioorg Med Chem Lett 24:3997–4000
Acknowledgments
We thank Prof. Tadashi Mizoguchi of Ritsumeikan University for his assistance in HPLC analyses. This work was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Artificial Photosynthesis (AnApple)” (No. 24107002) (to HT) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Anastasios Melis.
Rights and permissions
About this article
Cite this article
Teramura, M., Harada, J. & Tamiaki, H. In vitro stereospecific hydration activities of the 3-vinyl group of chlorophyll derivatives by BchF and BchV enzymes involved in bacteriochlorophyll c biosynthesis of green sulfur bacteria. Photosynth Res 130, 33–45 (2016). https://doi.org/10.1007/s11120-016-0220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-016-0220-7