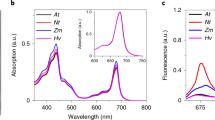
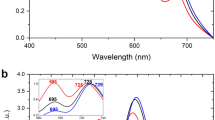
Abstract
In the sunlight-fluctuating environment, plants often encounter both light-deficiency and light-excess cases. Therefore, regulation of light harvesting is absolutely essential for photosynthesis in order to maximize light utilization at low light and avoid photodamage of the photosynthetic apparatus at high light. Plants have developed a series of strategies of light-harvesting regulation during evolution. These strategies include rapid responses such as leaf movement and chloroplast movement, state transitions, and reversible dissociation of some light-harvesting complex of the photosystem II (LHCIIs) from PSII core complexes, and slow acclimation strategies such as changes in the protein abundance of light-harvesting antenna and modifications of leaf morphology, structure, and compositions. This review discusses successively these strategies and focuses on the rapid change in antenna size, namely reversible dissociation of some peripheral light-harvesting antennas (LHCIIs) from PSII core complex. It is involved in protective role and species dependence of the dissociation, differences between the dissociation and state transitions, relationship between the dissociation and thylakoid protein phosphorylation, and possible mechanism for thermal dissipation by the dissociated LHCIIs.





Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine triphosphate
- A net (or P n):
-
Net photosynthetic rate
- Chl:
-
Chlorophyll
- CP24:
-
Lhcb6 (24 kDa) protein–pigment complex, a minor LHCII
- CP26:
-
Lhcb5 (26 kDa) protein–pigment complex, a minor LHCII
- CP29:
-
Lhcb4 (29 kDa) protein–pigment complex, a minor LHCII
- CP43:
-
A core antenna complex of PSII
- C2S2M2N2 :
-
PSII supercomplex, a dimeric core complex with six different type (S, M, N, for strong or tight bound, middle-intensity bound, and loose bound, respectively) LHCII trimers
- Cyt b 6 f :
-
Cytochrome b 6 f complex
- D1:
-
D1 protein of the photosystem II core complex
- D2:
-
D2 protein of the photosystem II core complex
- ΔpH:
-
Trans-thylakoid membrane proton gradient
- ELIP:
-
Early light-induced protein
- ETC:
-
Electron transport chain
- F 685 :
-
Chlorophyll fluorescence emissions peaked at 685 nm
- F 735 :
-
Chlorophyll fluorescence emissions peaked at 735 nm
- FSBA:
-
5′-p-Fluorosulfonylbenzoyl adenosine
- F v/F m :
-
Maximal or potential photochemical efficiency of photosystem II in dark-adapted leaves
- HLIP:
-
High light-induced protein
- LHC:
-
Light-harvesting complex
- LHCII:
-
Light-harvesting complex of the photosystem II
- LHCII-L:
-
Loose bound LHCII
- LHCII-M:
-
Middle-intensity bound LHCII
- LHCII-S:
-
Strong bound LHCII
- LHCSR:
-
Light-harvesting complex of green algae
- Lut:
-
Lutein
- NPQ:
-
Non-photochemical quenching of chlorophyll fluorescence or excitation energy
- P680:
-
Chlorophyll a molecule of the PSII reaction center
- PHOT:
-
Phototropin
- PGR5:
-
Proton gradient regulator 5
- PGRL1:
-
Ferredoxin–plastoquinone reductase 1
- PQ:
-
Plastoquinone
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- PsbS:
-
PsbS protein, a subunit of PSII
- Phy:
-
Phytochrome
- qE :
-
Chlorophyll fluorescence quenching or thermal dissipation dependent on trans-thylakoid membrane proton gradient or feedback de-excitation
- qI :
-
Photoinhibitory quenching
- qT :
-
State transition-dependent quenching
- qZ :
-
Zeaxanthin-dependent quenching
- RC:
-
Reaction center
- STN7:
-
Protein kinase for LHCII protein phosphorylation in higher plants
- STN8:
-
Protein kinase for PSII core protein phosphorylation
- Stt7:
-
Protein kinase from green algae
- TM:
-
Thylakoid membrane
- Zea:
-
Zeaxanthin
References
Ahn TK, Avenson TJ, Ballottari M, Cheng Y-C, Niyogi KK, Bassi R, Fleming GR (2008) Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science 320:794–797
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Allmer J, Naumann B, Markert C, Zhang M, Hippler M (2006) Mass spectrometric genomic data mining: novel insights into bioenergetic pathways in Chlamydomonas reinhardtii. Proteomics 6:6207–6220
Allorent G, Tokutsu R, Roach T, Peers G, Cardol P, Girard-Bascou J, Seigneurin-Berny D, Petroutsos D, Kuntz M, Breyton C, Frank F, Wollman F-A, Niyogi KK, Krieger-Liszkay A, Minagawa J, Finazzi G (2013) A dual strategy to cope with high light in Chlamydomonas reinhardtii. Plant Cell 25:545–557
Anderson JM (1986) Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol 37:93–136
Anderson JM (1999) Insights into the consequences of grana staking of thylakoid membranes in vascular plants: a personal perspective. Aust J Plant Physiol 26:625–639
Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organization in sun/shade acclimation. Aust J Plant Physiol 15:11–15
Anderson JM, Park Y-I, Chow WS (1997) Photoinactivation and photoprotection of photosystem II in nature. Physiol Plant 100:214–223
Aro E-M, Rokka A, Vener AV (2004) Determination of phosphoproteins in higher plant thylakoids. In: Carpentier R (ed) Methods in molecular biology, vol 274. Humana Press, Inc., Totowa, pp 271–285
Augustynowicz J, Gabrys H (1999) Chloroplast movements in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant Cell Environ 22:1239–1248
Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, Bassi R, Fleming GR (2008) Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 283:3550–3558
Avenson TJ, Ahn TK, Niyogi KK, Ballottari M, Bassi R, Fleming GR (2009) Lutein can act as a switchable charge transfer quencher in the CP26 light-harvesting complex. J Biol Chem 283:2830–2835
Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282:8947–8958
Ballottari M, Girardon J, Betterle N, Morosinotto T, Bassi R (2010) Identification of the chromophores involved in aggregation-dependent energy quenching of the monomeric photosystem II antenna protein Lhcb5. J Biol Chem 285:28309–28321
Barker DH, Seaton GGR, Robinson SA (1997) Internal and external photoprotection in developing leaves of the CAM plant Cotyledon orbiculata. Plant Cell Environ 20:617–624
Bassi RL, Giacometti GM, Simpson DJ (1988) Changes in the organization of stroma membranes induced by in vivo state-1-state-2 transition. Biochim Biophys Acta 935:152–165
Bassi RL, Rigoni F, Giacometti GM (1990) Chlorophyll binding proteins with antenna function in higher plants and green algae. Photochem Photobiol 52:1187–1206
Belgio E, Johnson MP, Juric S, Ruban AV (2012) Higher plant photosystem II light-harvesting antenna, not the reaction center, determines the excited-state lifetime—both the maximum and the nonphotochemically quenched. Biophys J 102:2761–2771
Belgio E, Kapitonova E, Chemeliov J, Duffy CDP, Ungerer P, Valkunas L, Ruban AV (2014) Economic photoprotection in photosystem II that retains a complete light-harvesting system with slow energy traps. Nat Commun. doi:10.1038/ncomms5433
Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433:892–895
Bennett J (1977) Phosphorylation of chloroplast membrane polypeptides. Nature 269:344–346
Bennett J (1991) Protein phosphorylation in green plant chloroplasts. Annu Rev Plant Physiol Plant Mol Biol 42:281–311
Bergantino E, Segalia A, Brunetta A, Teardo E, Rogoni F, Giacometti GM, Szabo I (2003) Light- and pH-dependent structural changes in the PsbS protein of photosystem II. Proc Natl Acad Sci USA 100:15265–15270
Betterle N, Ballottari M, Zorzan S, de Bianchi S, Cazzaniga S, DallOsto L, Morosinotto T, Bassi R (2009) Light-induced dissociation of an hetero-oligomer is needed for non-photochemical quenching induction. J Biol Chem 284:15255–15266
Björkman O, Demmig-Adams B (1994) Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, pp 17–47
Bode S, Quentmeier CC, Liao PN, Hafi N, Barros T, Wilk L, Bittner F, Walla PJ (2009) On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc Natl Acad Sci USA 106:12311–12316
Boekema EJ, Van Roon H, Van Breemen JF, Dekker JP (1999) Supramolecular organization of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Eur J Biochem 266:444–452
Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437:1179–1182
Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidos. Biochim Biophys Acta 189:366–383
Bonente G, Howes BD, Caffarri S, Smulevich G, Bassi R (2008a) Interactions between the photosystem II subunit PsbS and xanthophylls studied in vivo and in vitro. J Biol Chem 283:8434–8445
Bonente G, Passarini F, Cazzaniga S, Mancone C, Buia MC, Tripodi M, Bassi R, Caffarri S (2008b) The occurrence of the psbS gene product in Chlamydomonas reinhardtii and in other photosynthetic organisms and its correlation with energy quenching. Photochem Photobiol 84:1359–1370
Cai S-Q, Xu D-Q (2002) Light intensity-dependent reversible down-regulation and irreversible damage of PSII in soybean leaves. Plant Sci 163:847–853
Canaani O (1990) Photoacoustic studies on the dependence of state transitions on grana stacking. Photosynth Res 25:225–232
Chen Y, Xu D-Q (2006) Two patterns of leaf photosynthetic response to irradiance transition from saturating to limiting one in some plant species. N Phytol 169:789–798
Chen Y, Xu D-Q (2007) Species-dependence of the pattern of plant photosynthetic rate response to light intensity transition from saturating to limiting one. J Plant Physiol Mol Biol 33:538–546 (in Chinese with an abstract)
Chen Y, Xu D-Q (2009) Dissociation of photosystem II light-harvesting complex (LHC II) from the reaction center complex induced by saturating white irradiation differs from the transition from state 1 to state 2 induced by weak red irradiation. Acta Bot Yunnanica 31:67–74
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schunemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285
Dall’Osto L, Caffarri S, Bassi R (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17:1217–1232
Darwin C (1880) The power of movement in plants. John Murray, London
Dau H, Hansen UP (1988) The involvement of spillover in State 1–State 2 transitions in intact leaves at low light intensities. Biochim Biophys Acta 934:156–159
Daum B, Nicastro D, Austin JII, McIntosh JR, Kuhlbrandt W (2010) Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell 22:1299–1312
de Bianchi S, Betterle N, Kouril R, Cazzaniga S, Boekma E, Bassi R, Dall’Osto L (2011) Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant J 23:2659–2679
DeBlasio SL, Mullen JL, Luesse DR, Hangarter RP (2003) Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Physiol 133:1471–1479
Dekker JP, Boekema EJ (2005) Supermolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706:12–39
Delosme R, Olive J, Wollman FA (1996) Changes in light energy distribution upon state transitions: an in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta 1273:150–158
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. N Phytol 172:11–21
Depege N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299:1572–1575
Dietzel L, Brautigam K, Pfannschmidt T (2008) Photosynthetic acclimation: state transitions and adjustment of photosystem stoichiometry–functional relationships between short-term and long-term light quality acclimation in plants. FEBS J 275:1080–1088
Drop B, Webber-Birungi M, Yadav SKN, Filipowicz-Szymanska A, Fusetti F, Boekema EJ, Croce R (2014) Light-harvesting complex (LHCII) and its supermolecular organization in Chlamydomonas reinhardtii. Biochim Biophys Acta 1837:63–72
Eberhard S, Finazzi G, Wollman F-A (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515
Ehleringer JR, Björkman O (1978) Pubescence and leaf spectral characteristics of a desert shrub Encelia farinose. Oecologia 36:151–162
Ehleringer JR, Mooney HA, Glumon SL, Rundel PW (1981) Parallel evolution of leaf pubescence in Encelia in coastal deserts of North and South America. Oecologia 71:318–320
Elrad D, Niyogi KK, Grossman AR (2002) A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14:1801–1816
Engelken J, Brinkmann H, Adamska I (2010) Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein super-family. BMC Evol Biol 10:233
Engelken J, Funk C, Adamska I (2012) The extended light-harvesting complex (LHC) protein superfamily: classification and evolutionary dynamics. In: Burnap RL, Vermaas WFJ (eds) Functional genomics and evolution of photosynthetic systems. Springer, Dordrecht, pp 265–284
Ferl RJ (1996) 14-3-3 proteins and signal transduction. Annu Rev Plant Physiol Plant Mol Biol 47:49–73
Finazzi G, Forti G (2004) Metabolic flexibility of the green alga Chlamydomonas reinhardtii as revealed by the link between state transitions and cyclic electron flow. Photosynth Res 82:327–338
Finazzi G, Furia A, Barbagallo RP, Forti G (1999) State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii. Biochim Biophys Acta 1413:117–129
Finazzi G, Rappaport F, Furia A, Fleischmann M, Rochaix J-D, Zito F, Forti G (2002) Improvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep 3:280–285
Finazzi G, Johnson GN, Dall’Osto L, Zito F, Bonente G, Bassi R, Wollman F-A (2006) Nonphotochemical quenching of chlorophyll fluorescence in Chlamydomonas reinhardtii. Biochemistry 45:1490–1498
Floris M, Bassi R, Robaglia C, Alboresi A, Lanet E (2013) Post-transcriptional control of light-harvesting genes expression under light stress. Plant Mol Biol 82:147–154
Fork DC, Satoh K (1986) The control by state transitions of the distribution of excitation energy in photosynthesis. Annu Rev Plant Physiol 37:335–361
Frank HA, Bautista JA, Josue JS, Young AJ (2000) Mechanism of nonphotochemical quenching in green plants: energies of the lowest excited singlet states of violaxanthin and zeaxanthin. Biochemistry 39:2831–2837
Frigerio S, Campoli C, Zorzan S, Fantoni LI, Crosatti C, Drepper F, Haehnel W, Cattivelli L, Morosinotto T, Bassi R (2007) Photosynthetic antenna size in higher plants is controlled by the plastoquinone redox state at the post-transcriptional rather than transcriptional level. J Biol Chem 282:29457–29469
Gal A, Zer H, Ohad I (1997) Redox-controlled thylakoid protein phosphorylation. News and views. Physiol Plant 100:863–868
Gallagher S, Short TW, Ray PM, Pratt LH, Briggs WR (1988) Light-mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc Natl Acad Sci USA 85:8003–8007
Gamon JA, Pearcy RW (1989) Leaf movement, stress avoidance and photosynthesis in Vitis californica. Oecologia 79:475–481
Goral TK, Johnson MP, Duffy CDP, Brain APR, Ruban AV, Mullineaux CW (2012) Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J 69:289–301
Gould KS (2010) Muriel Wheldale Onslow and the rediscovery of anthocyanin function in plants. In: Santos-Buelga C, Escribano-Bailon MT, Lattanzio V (eds) Recent advances in polyphenol research, vol 2. Wiley-Blackwell Publishing Ltd., Singapore, pp 206–225
Grace SC, Logan BA, Adams WW (1998) Seasonal differences in foliar content of chlorogenic acid, a phenyl propanoid antioxidant, in Mahonia repens. Plant Cell Environ 21:513–521
Harada A, Sakai T, Okada K (2003) Phot 1 and phot 2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100:8583–8588
Haupt W, Scheuerlein R (1990) Chloroplast movement. Plant Cell Environ 13:595–614
Havaux M, Guedeney G, He Q, Grossman AR (2003) Elimination of high-light-inducible polypeptides related to eukaryotic chlorophyll a/b-binding proteins results in aberrant photoacclimation in Synechocystis PCC6803. Biochim Biophys Acta 1557:21–33
Heddad M, Noren H, Reiser V, Dunaeva M, Andersson B, Adamska I (2006) Differential expression and localization of early light-induced proteins in Arabidopsis. Plant Physiol 142:75–87
Herbstova M, Tietz S, Kinzel C, Turkina MV, Kirchhoff H (2012) Architectural switch in plant photosynthetic membranes induced by light stress. Proc Natl Acad Sci USA 109:20130–20135
Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin–plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49:511–523
Holleboom C-P, Walla PJ (2014) The back and forth of energy transfer between carotenoids and chlorophylls and its role in the regulation of light harvesting. Photosynth Res 119:215–221
Holt NE, Zigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Holzwarth AR, Miloslavina Y, Nilkens M, Jahns P (2009) Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem Phys Lett 483:262–267
Hong S-S, Xu D-Q (1999a) Reversible inactivation of PS II reaction centers and the dissociation of LHC II from PS II complex in soybean leaves. Plant Sci 147:111–118
Hong S-S, Xu D-Q (1999b) Light-induced increase in initial chlorophyll fluorescence Fo level and the reversible inactivation of PSII reaction centers in soybean leaves. Photosynth Res 61:269–280
Hong S-S, Hong T, Jiang H, Xu D-Q (1999) Changes in the non-photochemical quenching of chlorophyll fluorescence in flag leaves of wheat during aging. Photosynthetica 36:621–625
Horton P (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Philos Trans R Soc B 367:3455–3465
Horton P, Hague A (1988) Studies on the induction pf chlorophyll fluorescence in isolated barley protoplasts. IV. Resolution of non-photochemical quenching. Biochim Biophys Acta 932:107–115
Horton P, Ruban AV (2004) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56:365–373
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Horton P, Ruban AV, Wentworth M (2000) Allosteric regulation of the light-harvesting system of photosystem II. Philos Trans R Soc B 355:1361–1370
Horton P, Wentworth M, Ruban AV (2005) Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579:4201–4206
Horton P, Johnson MP, Perez-Bueno M, Kiss AZ, Ruban AV (2008) Does the structure and macroorganization of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J 275:1069–1079
Hou CX, Rintamaki E, Aro E-M (2003) Ascorbate-mediated LHCII protein phosphorylation: LHCII kinase regulation in light and in darkness. Biochemistry 42:5828–5836
Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278:2120–2123
Huang W, Zhang S-B, Cao K-F (2012) Evidence for leaf fold to remedy the deficiency of physiological photoprotection for photosystem II. Photosynth Res 110:185–191
Inada S, Ohgishi M, Mayama T, Okada K, Sakai T (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16:887–896
Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464:1210–1213
Jahns P, Holzwarth AR (2012) The role of the xanthophylls cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Jansson S (2006) A protein family saga: from photoprotection to light-harvesting (and back?). In: Demmig-Adams B, William W, Adams WW III, Mattoo AK (eds) Photoprotection, photoinhibition, gene regulation and environment. Springer, Dordrecht, pp 145–153
Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410:952–954
Johnson MP, Ruban AV (2009) Photoprotective energy dissipation in higher plants involves alteration of the excited state energy of the emitting chlorophyll (s) in the light harvesting antenna II (LHCII). J Biol Chem 284:23592–23601
Johnson MP, Davison PA, Ruban AV, Horton P (2008) The xanthophylls cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana. FEBS Lett 582:259–263
Johnson MP, Goral TK, Duffy CDP, Brain APR, Mullineaux CW, Ruban AV (2011) Photoprotective energy dissipation involves the reorganization of photosystem II light harvesting complexes in the grana membranes of higher plant chloroplasts. Plant Cell 23:1468–1479
Kadota A, Sato Y, Wada M (2000) Intracellular chloroplast photorelocation in the moss Physcomitrella patens is mediated by phytochrome as well as by a blue-light receptor. Planta 210:932–937
Kagawa T (2003) The phototropin family as photoreceptors for blue light-induced chloroplast relocation. J Plant Res 116:77–82
Kagawa T, Wada M (2004) Velocity of chloroplast avoidance movement is fluence rate dependent. Photochem Photobiol Sci 3:592–595
Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291:2138–2141
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plant. Nature 420:829–832
Kawai H, Kanegae T, Christensen S, Kiyosue T, Sato Y, Imaizumi T, Kadota A, Wada M (2003) Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421:287–290
Kereiche S, Kiss AZ, Kouril R, Boekema EJ, Horton P (2010) The PsbS protein controls the macro-organization of photosystem II complexes in the grana membranes of higher plant chloroplasts. FEBS Lett 584:759–764
Khatoon M, Inagawa K, Pospisil P, Yamashita A, Yoshioka M, Lundin B, Horie J, Morita N, Jajoo A, Yamamoto Y (2009) Quality control of photosystem II: thylakoid unstacking is necessary to avoid further damage to the D1 protein and to facilitate D1 degradation under light stress in spinach thylakoids. J Biol Chem 284:25343–25352
Kim S, Pichersky E, Yocum CF (1994) Topological studies of spinach 22 kDa protein of Photosystem II. Biochim Biophys Acta 1188:339–348
Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and Phot2 mediate blue light regulation of stomatal opening. Nature 414:656–660
Kleine T, Kindgren P, Benedict C, Hendrickson L, Strand Å (2007) Genome wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 144:1391–1406
Knieb E, Salomon M, Rudiger W (2004) Tissue-specific and subcellular localization of phototropin determined by immuno-blotting. Planta 218:843–851
Koller D (1990) Light-driven leaf movements. Plant Cell Environ 13:615–632
Kondo A, Kaikawa J, Funaguma T, Ueno O (2004) Clumping and dispersal of chloroplasts in succulent plants. Planta 219:500–506
Kouril R, Zygadlo A, Arteni AA, de Wit CD, Dekker JP, Jensen PE, Scheller HV, Boekema EJ (2005) Structural characterization of a complex of photosystem I and light-harvesting complex II in Arabidopsis thaliana. Biochemistry 44:10935–10940
Kouril R, Dekker JP, Boekema EJ (2012) Supramolecular organization of photosystem II in green plants. Biochim Biophys Acta 1817:2–12
Kouril R, Wientjes E, Bultema JB, Croce R, Boekema EJ (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta 1827:411–419
Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316:715–719
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kreslavski VD, Zorina AA, Los DA, Fomina IR, Allakhverdiev SI (2013) Molecular mechanisms of stress resistance of photosynthetic machinery. In: Rout GR, Das AB (eds) Molecular stress physiology of plants. Springer, Dordrecht, pp 21–51
Krüger TPJ, Novoderezhkin VI, Hioaia C, van Grondelle R (2010) Fluorescence spectral dynamics of single LHCII trimers. Biophys J 98:3093–3101
Krüger TP, Ilioaia C, Johnson MP, Ruban AV, Papagiannakis E, Horton P, van Grondelle R (2012) Controlled disorder in plant light-harvesting complex II explains its photoprotective role. Biophys J 102:2669–2676
Kühlheim C, Ågren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297:91–93
Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Li X-P, Gilmore AM, Niyogi KK (2002) Molecular and global time-resolved analysis of a psbS gene dosage effect on pH- and xanthophyll cycle-dependent non-photochemical quenching in photosystem II. J Biol Chem 277:33590–33597
Liakopoulos G, Nikolopoulos D, Klouvatou A, Vekkos K-A, Manetas Y, Karabourniotis G (2006) The photoprotective role of epidermal anthocyanins and surface pubescence in young leaves of grapevine (Vitis vinifera). Ann Bot 98:257–265
Liao Y, Xu D-Q (2007) Novel evidence for reversible dissociation of light-harvesting complex II from photosystem II reaction center complex induced by saturating light illumination in soybean leaves. J Integr Plant Biol 49:523–530
Liao P-N, Holleboom C-P, Wilk L, Kühlbrandt W, Walla PJ (2010) Correlation of Car S1 → Chl with Chl → Car S1 energy transfer supports the excitonic model in quenched light harvesting complex II. J Phys Chem B 114:15650–15655
Liu Z, Chang W (2008) Structure of the light-harvesting complex II. In: Fromme P (ed) Photosynthetic protein complexes: a structural approach. Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim, pp 217–242
Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
Ludlow MM, Björkman O (1984) Paraheliotropic leaf movement in Siratro as a protective mechanism against drought-induced damage to primary photosynthetic reactions: damage by excessive light and heat. Planta 161:505–518
Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408:613–615
Lunde C, Jensen PE, Rosgaard L, Haldrup A, Gilpin MJ, Scheller HV (2003) Plants impaired in state transition can to a large degree compensate for their defect. Plant Cell Physiol 44:44–54
Malkin S, Telfer A, Barber J (1986) Quantitative analysis of State 1–State 2 transitions in intact leaves using modulated fluorimetry—evidence for changes in the absorption cross-section of the two photosystems during state transitions. Biochim Biophys Acta 848:48–57
McKim SM, Durnford DG (2006) Translational regulation of light-harvesting complex expression during photoacclimation to high-light in Chlamydomonas reinhardtii. Plant Physiol Biochem 44:857–865
Melis A (1991) Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta 1058:87–106
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59:3903–3911
Miloslavina Y, Wehner A, Lambrev PH, Wientjes E, Reus M, Garab G, Croce R, Holzwarth AR (2008) Far-red fluorescence: a direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett 582:3625–3631
Montane MH, Kloppstech K (2000) The family of light-harvesting-related proteins (LHCs, ELIPs, HLIPs): was the harvesting of light their primary function? Gene 258:1–8
Mooney HA, Ehleringer JR, Björkman O (1977) The energy balance of leaves of the evergreen shrub (Atriplex hymenolytra). Oecologia 29:301–310
Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286:961–964
Muller MG, Lambrev P, Reus M, Wientjes E, Croce R, Holzwarth AR (2010) Singlet energy dissipation does not involve energy transfer to carotenoids. Chem Phys Chem 11:1289–1296
Murata N (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172:241–251
Murchie EH, Horton P (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20:438–448
Nilkens M, Kress E, Lambrev PH, Miloslavina Y, Muller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady state conditions in Arabidopsis. Biochim Biophys Acta 1797:466–475
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Niyogi KK (2005) Is PsbS the site of nonphotochemical quenching in photosynthesis? J Exp Bot 56:375–382
Palmer JM, Short TW, Gallagher S, Briggs WR (1993) Blue light-induced phosphorylation of a plasma membrane-associated protein in Zea mays L. Plant Physiol 102:1211–1218
Park YI, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111:867–875
Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban A (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436:134–137
Pastenes C, Pimentel P, Lillo J (2005) Leaf movements and photoinhibition in relation to water stress in field-grown beans. J Exp Bot 56:425–433
Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462:518–521
Pesaresi P, Hertle A, Pribl M, Kleine T, Wagner R, Strissel H, Ihnatowicz A, Bonardi V, Scharfenberg M, Schneider A, Pfannschmidt T, Leister D (2009) Arabidopsis STN7 kinase provides a link between short- and long term photosynthetic acclimation. Plant Cell 21:2402–2423
Rintamaki E, Martinsuo P, Pursiheimo S, Aro E-M (2000) Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin–thioredoxin system in chloroplasts. Proc Natl Acad Sci USA 97:11644–11649
Rochaix J-D (2014) Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 65:287–309
Ruban AV (2013) Adaptations of the photosynthetic membrane to light. In: The photosynthetic membrane: molecular mechanisms and biophysics of light harvesting. Wiley, Singapore, pp 197–240
Ruban AV (2015) Evolution under the sun: optimizing light harvesting in photosynthesis. J Exp Bot 66:7–23
Ruban AV, Johnson MP (2009) Dynamics of the photosystems cross-section associated with the state transition in higher plants. Photosynth Res 99:173–183
Ruban AV, Trach VV (1991) Heat-induced reversible changes in photosystem 1 absorption cross-section of pea chloroplasts and sub-chloroplast preparations. Evidence from excitation fluorescence spectra. Photosynth Res 29:157–169
Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–578
Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Sakai T, Kagawa T, Kasahara M, Swartz TE, Christien JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph 1 and npl 1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98:6969–6974
Sakamoto K, Briggs WR (2002) Cellular and subcellular localization of phototropin 1. Plant Cell 14:1723–1735
Salonen M, Aro E-M, Rintamäki E (1998) Reversible phosphorylation and turnover of the D1 protein under various redox states of photosystem II induced by low temperature photoinhibition. Photosynth Res 58:143–151
Samol I, Shapiguzov A, Ingelsson B, Fucile G, Crevecoeur M, Vener AV, Rochaix J-D, Goldschmidt-Clermont M (2012) Identification of a photosystem II phosphatase involved in light acclimation in Arabidopsis. Plant Cell 24:2596–2609
Samson G, Bruce D (1995) Complementary changes in absorption cross-sections of photosystem I and II due to phosphorylation and Mg2+-depletion in spinach thylakoids. Biochim Biophys Acta 1232:21–26
Sato Y, Kadota A (2006) Chloroplast movements in response to environmental signals. In: Wise RR, Hoober JK (eds) The structure and function of plastids. Springer, Dordrecht, pp 527–537
Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix J-D, Vener AV, Goldschmidt-Clermont M (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci USA 107:4782–4787
Sharma VK, Jain PK, Maheshwari SC, Khurana JP (1997) Rapid blue-light-induced phosphorylation of plasma-membrane-associated proteins in wheat. Phytochemistry 44:775–780
Sharon Y, Beer S (2008) Diurnal movements of chloroplasts in Halophila stipulacea and their effect on PAM fluorometric measurements of photosynthetic rates. Aquat Bot 88:273–276
Sharon Y, Dishon G, Beer S (2011) The effects of UV radiation on chloroplast clumping and photosynthesis in the seagrass Halophila stipulacea grown under high-PAR conditions. J Mar Biol 2011:1–6
Snyders S, Kohorn BD (2001) Disruption of thylakoid-associated kinase 1 leads to alteration of light harvesting in Arabidopsis. J Biol Chem 276:32169–32176
Standfuss J, Kühlbrandt W (2004) The three isoforms of light-harvesting complex II: spectroscopic features, trimer formation, and functional roles. J Biol Chem 279:36884–36891
Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P (2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100:1456–1461
Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 421:79–83
Sundby C, Andersson B (1985) Temperature-induced reversible migration along the thylakoid membrane of photosystem II regulates its association with LHC-II. FEBS Lett 191:24–28
Takagi S (2003) Actin-based photo-orientation movement of chloroplasts in plant cells. J Exp Biol 206:1963–1969
Takahashi S, Badger MR (2011) Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16:53–60
Takahashi H, Iwai M, Takahashi Y, Minagawa J (2006) Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103:477–482
Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4:1954
Tan X-X, Xu D-Q, Shen Y-K (1998) Both spillover and light absorption cross-section changes are involved in the regulation of excitation energy distribution between the two photosystems during state transitions in wheat leaf. Photosynth Res 56:95–102
Teramoto H, Nakamori A, Minagawa J, Ono T (2002) Light intensity-dependent expression of Lhc gene family encoding light-harvesting chlorophyll-a/b proteins of photosystem II in Chlamydomonas reinhardtii. Plant Physiol 130:325–333
Teramoto H, Itoh T, Ono T (2004) High-intensity-light-dependent and transient expression of new genes encoding distant relatives of light-harvesting chlorophyll-a/b proteins in Chlamydomonas reinhardtii. Plant Cell Physiol 45:1221–1232
Tikkanen M, Grieco M, Kangasjarvi S, Aro E-M (2010) Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 152:723–735
Tikkanen M, Grieco M, Aro E-M (2011) Novel insights into plant light-harvesting complex II phosphorylation and ‘state transition’. Trends Plant Sci 16:126–131
Tokutsu R, Minagawa J (2013) Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 110:10016–10021
Turkina MV, Kargul J, Blanco-Rivero A, Villarejo A, Barber J, Vener AV (2006) Environmentally modulated phosphoproteome of photosynthetic membranes in the green alga Chlamydomonas reinhardtii. Mol Cell Proteomics 5:1412–1425
Ueda M, Nakamura Y (2010) Plant phenolic compounds controlling leaf movement. In: Santos-Buelga C, Escribano-Bailon MT, Lattanzio V (eds) Recent advances in polyphenol research. Wiley-Blackwell Publishing Ltd., Singapore, pp 226–237
Vallon O, Bulte L, Dainese P, Olive J, Bassi R, Wollman FA (1991) Lateral redistribution of cytochrome b 6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci USA 88:8262–8266
Van Amerongen H, Croce R (2013) Light harvesting in photosystem II. Photosynth Res 116:251–263
Veeranjameyulu K, Charland M, Charlebois D, Leblanc RM (1991) Photoacoustic study of changes in the energy storage of Photosystem I and II during state 1–state 2 transitions. Plant Physiol 97:330–334
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Wang Q, Jantaro S, Lu B, Majeed W, Bailey M, He Q (2008) The high light-inducible polypeptides stabilize trimeric photosystem I complex under high light conditions in Synechocystis PCC 6803. Plant Physiol 147:1239–1250
Wientjes E, Drop B, Kouril R, Boekema EJ, Croce R (2013a) During state 1 to state 2 transition in Arabidopsis thaliana, the photosystem II supercomplex gets phosphorylated but does not disassemble. J Biol Chem 288:32821–32826
Wientjes E, van Amerongen H, Croce R (2013b) LHCII is an antenna of both photosystems after long-term acclimation. Biochim Biophys Acta 1827:420–426
Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20:3623–3630
Xu D-Q (2013) Regulation of light-harvesting. The science of photosynthesis (in Chinese with English contents). Science Press, Beijing, pp 289–305
Yakushevska AE, Keegstra W, Boekema EJ, Dekker JP, Andersson J, Jansson S, Ruban AV, Horton P (2003) The structure of photosystem II in Arabidopsis: localization of the CP26 and CP29 antenna complexes. Biochemistry 42:608–613
Zhang H-B, Xu D-Q (2003a) Role of light-harvesting complex 2 dissociation in protecting the photosystem 2 reaction centres against photodamage in soybean leaves and thylakoids. Photosynthetica 41:383–391
Zhang H-B, Xu D-Q (2003b) Different mechanisms for photosystem 2 reversible down-regulation in pumpkin and soybean leaves under saturating irradiance. Photosynthetica 41:177–184
Zhang H-B, Cai S-Q, Xu D-Q (2002) D1 protein phosphorylation/dephosphorylation alone has no effect on the electron transport activity of photosystem II in soybean leaves. Plant Sci 162:507–511
Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman FA (1999) The Qo site of cytochrome b 6 f complexes controls the activation of the LHCII kinase. EMBO J 18:2961–2969
Acknowledgments
The authors thank Drs. Xin-Xing Tan, Shuang-Song Hong, Shi-Qing Cai, Hai-Bo Zhang, and Yi Liao for contributions to this work. This work was supported by the grants under the State Key Basic Research and Development Plan (No. 2015CB150104 and 2009CB118504) and the National Basic Research Program of China (Project No. 2005CB121106). The authors are also grateful to Professor Yun-Kang Shen for critically reading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, DQ., Chen, Y. & Chen, GY. Light-harvesting regulation from leaf to molecule with the emphasis on rapid changes in antenna size. Photosynth Res 124, 137–158 (2015). https://doi.org/10.1007/s11120-015-0115-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0115-z