Abstract
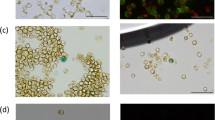
Chaetoceros gracilis belongs to the centric diatoms, and has recently been used in basic research on photosynthesis. In addition, it has been commercially used in fisheries and is also attracting interest as a feedstock for biofuels production and biorefinery. In this study, we developed an efficient genetic transformation system for C. gracilis. The diatom cells were transformed via multi-pulse electroporation using plasmids containing various promoters to drive expression of the nourseothricin acetyltransferase gene (nat) as a selectable marker. The transformation efficiency reached ~400 positive transgenic clones per 108 recipient cells, which is the first example of successful transformation with electroporation in a centric diatom species. We further produced two expression vectors: the vector pCgLhcr5p contains the light-dependent promoter of a fucoxanthin chlorophyll a/c binding protein gene and the vector pCgNRp contains the inducible promoter of a nitrate reductase gene to drive the expression of introduced genes. In both vectors, an acetyl-CoA acetyltransferase promoter drives nat gene expression for antibiotic selection. Stable integration and expression of reporter genes, such as the firefly luciferase and green fluorescent protein Azami–Green genes, were observed in transformed C. gracilis cells. This efficient and stable transformation system for C. gracilis will enable both functional analysis of diatom-specific genes and strain improvement for further biotechnological applications.







Similar content being viewed by others
References
Apt KE, Kroth-Pancic PG, Grossman AR (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol Gen Genet 252:572–579
Araujo GS, Matos LJ, Gonçalves LR, Fernandes FA, Farias WR (2011) Bioprospecting for oil producing microalgal strains: evaluation of oil and biomass production for ten microalgal strains. Bioresour Technol 102:5248–5250
Bhaya D, Grossman A (1993) Characterization of gene clusters encoding the fucoxanthin chlorophyll proteins of the diatom Phaeodactylum tricornutum. Nucleic Acids Res 21:4458–4466
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Chiu WL, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–333
Daboussi F, Leduc S, Maréchal A, Dubois G, Guyot V, Perez-Michaut C, Amato A, Falciatore A, Juillerat A, Beurdeley M, Voytas DF, Cavarec L, Duchateau P (2014) Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat Commun 5:3831. doi:10.1038/ncomms4831
De Riso V, Raniello R, Maumus F, Rogato A, Bowler C, Falciatore A (2009) Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res 37:e96
Dunahay TG, Jarvis EE, Roessler PG (1995) Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J Phycol 31:1004–1012
Falciatore A, Casotti R, Leblanc C, Abrescia C, Bowler C (1999) Transformation of nonselectable reporter genes in marine diatoms. Mar Biotechnol (NY) 1:239–251
Falkowski PG, Barber RT, Smetacek VV (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281:200–207
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Fischer H, Robl I, Sumper M, Kroger N (1999) Targeting and covalent modification of cell wall and membrane proteins heterologously expressed in the diatom Cylindrotheca fusiformis (Bacillariophyceae). J Phycol 35:113–120
Hasle GR, Syvertsen EE (1997) Marine Diatoms. In: Tomas CR (ed) Identifying marine phytoplankton. Academic Press, San Diego, CA, pp 5–385
Ikeda Y, Komura M, Watanabe M, Minami C, Koike H, Itoh S, Kashino Y, Satoh K (2008) Photosystem I complexes associated with fucoxanthin-chlorophyll-binding proteins from a marine centric diatom, Chaetoceros gracilis. Biochim Biophys Acta 1777:351–361
Karasawa S, Araki T, Yamamoto-Hino M, Miyawaki A (2003) A green-emitting fluorescent protein from Galaxeidae coral and its monomeric version for use in fluorescent labeling. J Biol Chem 278:34167–34171
Levitan O, Dinamarca J, Hochman G, Falkowski PG (2014) Diatoms: a fossil fuel of the future. Trends Biotechnol 32:117–124
Miyagawa A, Okami T, Kira N, Yamaguchi H, Ohnishi K, Adachi M (2009) Research note: high efficiency transformation of the diatom Phaeodactylum tricornutum with a promoter from the diatom Cylindrotheca fusiformis. Phycol Res 57:142–146
Miyagawa-Yamaguchi A, Okami T, Kira N, Yamaguchi H, Ohnishi K, Adachi M (2011) Stable nuclear transformation of the diatom Chaetoceros sp. Phycol. Res. 59:113–119
Miyahara M, Aoi M, Inoue-Kashino N, Kashino Y, Ifuku K (2013) Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci Biotechnol Biochem 77:874–876
Nagao R, Ishii A, Tada O, Suzuki T, Dohmae N, Okumura A, Iwai M, Takahashi T, Kashino Y, Enami I (2007) Isolation and characterization of oxygen-evolving thylakoid membranes and photosystem II particles from a marine diatom Chaetoceros gracilis. Biochim Biophys Acta 1767:1353–1362
Nagao R, Moriguchi A, Tomo T, Niikura A, Nakajima S, Suzuki T, Okumura A, Iwai M, Shen JR, Ikeuchi M, Enami I (2010) Binding and functional properties of five extrinsic proteins in oxygen-evolving photosystem II from a marine centric diatom, Chaetoceros gracilis. J Biol Chem 285:29191–29199
Nagao R, Yokono M, Teshigahara A, Akimoto S, Tomo T (2014) Light-harvesting ability of the fucoxanthin chlorophyll a/c-binding protein associated with photosystem II from the Diatom Chaetoceros gracilis as revealed by picosecond time-resolved fluorescence spectroscopy. J Phys Chem B 118:5093–5100
Nelson DM, Tréguer PM, Brzezinski A, Leynaert A, Quéguiner B (1995) Production and dissolution of biogenic silica an the ocean: revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochemical Cycle 9:359–372
Niu YF, Yang ZK, Zhang MH, Zhu CC, Yang WD, Liu JS, Li HY (2012) Transformation of diatom Phaeodactylum tricornutum by electroporation and establishment of inducible selection marker. Biotechniques. doi:10.2144/000113881
Poulsen N, Kröger N (2005) A new molecular tool for transgenic diatoms. FEBS J. 272:3413–3423
Poulsen N, Chesley PM, Kroger N (2006) Molecular genetic manipulation of the diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 42:1059–1065
Prihoda J, Tanaka A, de Paula WB, Allen JF, Tirichine L, Bowler C (2012) Chloroplast-mitochondria cross-talk in diatoms. J Exp Bot 63:1543–1557
Ramachandra TV, Mahapatra DM, K B, Gordon R (2009) Milking diatoms for sustainable energy: biochemical engineering versus gasoline-secreting diatom solar panels. Ind Eng Chem Res 48:8769–8788
Reid BG, Flynn GC (1997) Chromophore formation in green fluorescent protein. Biochemistry 36:6786–6791
Rushforth SR, Johansen JR (1986) The inland Chaetoceros (Bacillariophyceae) species of North America. J Phycol 22:441–448
Scheffel A, Poulsen N, Shian S, Kröger N (2011) Nanopatterned protein microrings from a diatom that direct silica morphogenesis. Proc Natl Acad Sci USA 108:3175–3180
Wang Y, Cai J, Jiang Y, Jiang X, Zhang D (2013) Preparation of biosilica structures from frustules of diatoms and their applications: current state and perspectives. Appl Microbiol Biotechnol 97:453–460
Yamano T, Iguchi H, Fukuzawa H (2013) Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. J Biosci Bioeng 115:691–694
Zaslavskaia LA, Lippmeier JC, Kroth PG, Grossman AR, Apt KE (2000) Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol 36:379–386
Zhang C, Hu H (2013) High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Mar Genomics 16:63–66
Acknowledgments
We thank Dr. N. Poulsen and Dr. N. Kröger at the Technical University of Dresden, Germany, for the gift of the vectors pTpNR-GFP/fcpNat, and Dr. J. Hotta, Yamagata Univ. for his help in microscopic analysis of the diatoms. We also appreciate the excellent technical assistance of Ms. N. Inui. This work was supported by the Japan Science and Technology Agency, Advanced Low Carbon Technology Research and Development Program (to K. I., Y. Y., and Y. K.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ifuku, K., Yan, D., Miyahara, M. et al. A stable and efficient nuclear transformation system for the diatom Chaetoceros gracilis . Photosynth Res 123, 203–211 (2015). https://doi.org/10.1007/s11120-014-0048-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0048-y