Abstract
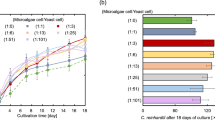
Four mutants of Chlamydomonas reinhardtii with defects in different components of the CO2 concentrating mechanism (CCM) or in Rubisco activase were grown autotrophically at high pCO2 and then transferred to low pCO2, in order to study the role of different components of the CCM on carbon allocation and elemental composition. To study carbon allocation, we measured the relative size of the main organic pools by Fourier Transform Infrared spectroscopy. Total reflection X-ray fluorescence was used to analyze the elemental composition of algal cells. Our data show that although the organic pools increased their size at high CO2 in all strains, their stoichiometry was highly homeostatic, i.e., the ratios between carbohydrates and proteins, lipid and proteins, and carbohydrates and lipids, did not change significantly. The only exception was the wild-type 137c, in which proteins decreased relative to carbohydrates and lipids, when the cells were transferred to low CO2. It is noticeable that the two wild types used in this study responded differently to the transition from high to low CO2. Malfunctions of the CCM influenced the concentration of several elements, somewhat altering cell elemental stoichiometry: especially the C/P and N/P ratios changed appreciably in almost all strains as a function of the growth CO2 concentration, except in 137c and the Rubisco activase mutant rca1. In strain cia3, defective in the lumenal carbonic anhydrase (CA), the cell quotas of P, S, Ca, Mn, Fe, and Zn were about 5-fold higher at low CO2 than at high CO2. A Principle Components Analysis showed that, mostly because of its elemental composition, cia3 behaved in a substantially different way from all other strains, at low CO2. The lumenal CA thus plays a crucial role, not only for the correct functioning of the CCM, but also for element utilization. Not surprisingly, growth at high CO2 attenuated differences among strains.




Similar content being viewed by others
References
Beardall J, Giordano M (2002) Ecological implications of microalgal and cyanobacterial CO2 mechanisms, and their regulation. Funct Plant Biol 29:335–347
Bi R, Arndt C, Sommer U (2012) Stoichiometric responses of phytoplankton species the interactive effect of nutrient supply ratios and growth rates. J Phycol 48:349–539
Brueggeman AJ, Gangadharaiah DS, Cserhati MF, Casero D, Weeksa DP, Ladung I (2012) Activation of the carbon concentrating mechanism by CO2 deprivation coincides with massive transcriptional restructuring in Chlamydomonas reinhardtii. Plant Cell 24:1860–1875
Domenighini A, Giordano M (2009) Fourier transform infrared spectroscopy of microalgae as a novel tool for biodiversity studies, species identification, and the assessment of water quality. J Phycol 45:522–531
Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham SS, McCauley E, Schulz KL, Siemann EV, Sterner RW (2000) Nutritional constraints in terrestrial and freshwater foodwebs. Nature 408:578–580
Fanesi A, Raven JA, Giordano M (2014) Growth rate affects the responses of the green alga Tetraselmis suecica to external perturbations. Plant Cell Environ 37:512–519
Fukuzawa H, Miura K, Ishizaki K, Kucho K, saito T, Kohinata T, Ohyama K (2001) Ccm1, a regulatory gene controlling the induction of a carbon concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci USA 98:5347–5352
Geider RJ, La Roche J (2002) Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17
Giordano M (2013) Homeostasis: an underestimated focal point of ecology and evolution. Plant Sci 211:92–101
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Gorman DS, Levine RP (1965) Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 54:1665–1669
Huppe HC, Turpin DH (1994) Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol 45:577–607
Kaffes A, Thoms S, Trimborn S, Rost B, Langer G, Richter K, Köhler A, Norici A, Giordano M (2010) Carbon and nitrogen fluxes in the marine coccolithophore Emiliania huxleyi grown under different nitrate concentrations. J Exp Mar Biol Ecol 393:1–8
Karlsson J, Clarke AK, Chen Z-Y, Hugghins SY, Park Y-I, Husic HD, Moroney JV, Samuelsson G (1998) A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17:1208–1216
Lowry OH (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma Y, Pollock SV, Xiao Y, Cunnusamy K, Moroney JV (2011) Identification of a novel gene, CIA6, required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol 156:884–896
Miura K, Yamano T, Yoshioka S, Kohinata T, Inoue Y, Taniguchi F, Asamizu E, Nakamura Y, Tabata S, Yamato KT, Ohyama K, Fukuzawa H (2004) Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 135:1595–1607
Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6:1251–1259
Moroney JV, Tolbert NE, Sears BB (1986) Complementation analysis of the inorganic Carbon Concentrating Mechanism of Chlamydomonas reinhardtii. Mol Genet Genomics 204:199–203
Moroney JV, Husic HD, Tolbert NE, Kitayama M, Manuel LJ, Togasaki RK (1989) Isolation and characterization of a mutant of Chlamydomonas reinhardtii deficient in the CO2 concentrating mechanism. Plant Physiol 89:897–903
Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee N (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosyth Res 109:133–149
Norici A, Bazzoni AM, Pugnetti A, Raven JA, Giordano M (2011) Impact of irradiance on the C allocation in the coastal marine diatom Skeletonema marinoi Sarno & Zingone. Plant Cell Environ 34:1666–1677
Palmucci M, Ratti S, Giordano M (2011) Ecological and evolutionary implications of carbon allocation in marine phytoplankton as a function of nitrogen availability: a fourier transform infrared spectroscopy approach. J Phycol 47:313–323
Pollock SV, Colombo SL, Prout DL, Godfrey AC, Moroney JV (2003) Rubisco activase is required for optimal photosynthesis in the green alga Chlamydomonas reinhardtii in a low-CO2 atmosphere. Plant Physiol 133:1854–1861
Portis AR (1995) The regulation of rubisco by rubisco activase. J Exp Bot 46:1285–1291
Ratti S, Morse D, Giordano M (2007) CO2 concentrating mechanisms of the potentially toxic dinoflagellate Protoceratium reticulatum (Dynophyceae, Gonyaulacales). J Phycol. 43:693–701
Raven JA (1997) CO2 concentrating mechanisms: a direct role for thylakoid lumen acidification? Plant Cell Environ 20:147–154
Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC (2005) Algae lacking carbon concentrating mechanisms. Can J Bot 83:879–890
Raven JA, Giordano M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109:281–296
Raven JA, Giordano M, Beardall J, Maberly SC (2012) Algal evolution in relation to atmospheric CO2: carboxylases, carbon concentrating mechanisms and carbon oxidation cycles. Philos Trans Roy Soc B 367:493–507
Raven JA, Beardall J and Giordano M (2014) Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynth Res. doi:10.1007/s11120-013-9962-7
Ruan Z (2013) Energy partitioning between the CO2 concentrating mechanism and N assimilation in the cyanobacterium Synechoccus UTEX 2380 repercussions on cell composition and stoichiometry. Ph.D Thesis, Università Politecnica delle Marche, Ancona
Salvucci ME, Ogren WL (1996) The mechanism of Rubisco activase: insights from studies of the properties and structure of the enzyme. Photosynth Res 47:1–11
Schnell RA, Lefebvre PA (1993) Isolation of the Chlamydomonas regulatory gene NIT2 by transposon tagging. Genetics 134:737–747
Sueoka N (1960) Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proc Natl Acad Sci USA 46(1):83–91
Umen JG, Goodenough UW (2001) Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev 15:1652–1661
Xiang Y, Zhang J, Weeks DP (2001) The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 98:5341–5346
Young EB, Beardall J (2003) Rapid ammonium- and nitrate-induced perturbations to Chl a fluorescence in nitrogen-stressed Dunaliella tertiolecta (Chlorophyta). J Phycol 39:332–342
Acknowledgments
CCM work in MG’s lab was funded by the Cariverona Foundation, by the Italian Ministry for Agriculture (MIPAF, Bioforme project), by the Italian Ministry of Foreign Affairs (MAE, Joint Italian-Israel Cooperation Program), and by the Assemble program of the European Union. The work in JVM’s lab was funded by the National Science Foundation and a subcontract from the University of Illinois. We wish to thank Elly Spijkerman for providing access to the instrumentation for the C and N quantification.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Memmola, F., Mukherjee, B., Moroney, J.V. et al. Carbon allocation and element composition in four Chlamydomonas mutants defective in genes related to the CO2 concentrating mechanism. Photosynth Res 121, 201–211 (2014). https://doi.org/10.1007/s11120-014-0005-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0005-9