Abstract
The fluorescence induction F(t) of dark-adapted chloroplasts has been studied in multi-turnover 1 s light flashes (MTFs). A theoretical expression for the initial fluorescence rise is derived from a set of rate equations that describes the sequence of transfer steps associated with the reduction of the primary quinone acceptor Q A and the release of photochemical fluorescence quenching of photosystem II (PSII). The initial F(t) rise in the hundreds of μs time range is shown to follow the theoretical function dictated by the rate constants of light excitation (k L) and release of donor side quenching (k si ). The bi-exponential function shows sigmoidicity when one of the two rate constants differs by less than one order of magnitude from the other. It is shown, in agreement with the theory, that the sigmoidicity of the fluorescence rise is variable with light intensity and mainly, if not exclusively, determined by the ratio between rate of light excitation and the rate constant of donor side quenching release.






Similar content being viewed by others
Abbreviations
- B(t):
-
Normalized area above rFv(t)
- DCMU:
-
3(3,4-Dichlorophenyl)-1,1-dimethylurea
- DSQ:
-
Donor side quenching
- FmS(M)TF :
-
Fluorescence level of system with 100% closed PSUs after S(M)TF excitation in dark-adapted state
- Fo:
-
Fluorescence level of system with 100% open PSUs in dark-adapted state
- rFv:
-
Relative variable fluorescence (F − Fo)/(Fm − Fo)
- k −1 :
-
Rate constant of radical pair recombination
- k AB :
-
Rate constant of Q −A oxidation
- k d :
-
Rate constant of non-radiative radical pair transfer
- k e :
-
Rate constant of Q A photoreduction (charge stabilization at acceptor side)
- k L :
-
Excitation rate of photosystem in light pulse
- k t :
-
Rate constant of photochemical trapping (charge separation) in PSII
- k w :
-
Rate constant of non-photochemical energy losses
- k yi,si :
-
Rate constant of P+- and Y +Z -reduction, respectively, fo′r OEC in S = S i -state (i = 0, …, 3)
- nFv:
-
Normalized variable fluorescence (F − Fo)/Fo
- q :
-
Fraction of RCs with Q −A
- q dsq :
-
Fraction of RCs in which acceptor- and donor side quenching is released
- MTF:
-
Multi-turnover flash (light pulse)
- OEC:
-
Oxygen evolving complex
- ODE:
-
Ordinary linear differential equation
- Φ otr :
-
Electron trapping efficiency of open RCs
- P680 (or P):
-
Mainstream electron donor of PSII
- Phe (or Ph):
-
Pheophytin, primary electron acceptor of PSII
- PSII:
-
Photosystem II
- PSU II:
-
Photosynthetic unit of PSII
- Q A :
-
Primary quinone acceptor of PSII
- Q B :
-
Secondary quinone acceptor of PSII
- RCII:
-
Reaction center of PSII
- STF:
-
Single turnover flash (excitation)
- TSTM:
-
Three-state trapping model
- YZ :
-
Secondary electron donor of PSII
References
Anderson JM, Melis A (1983) Localization of different photosystems in separate regions of chloroplast membranes. Proc Natl Acad Sci USA 80:745–749
Babcock G (1987) The photosynthetic evolving process. In: Amesz J (ed) New comprehensive biochemistry, vol 15. Elsevier, Amsterdam, pp 125–158
Belyaeva NT, Paschenko VZ, Renger G, Riznichenko Yu G, Rubin AB (2006) Application of photosystem II model for analysis of fluorescence induction curves in the 100 ns to 10 s time domain after excitation with a saturating light pulse. Biophysics (Translated from Biofizika) 51(6):976–990
Bernhardt K, Trissl H-W (1999) Theories for kinetics and yields of fluorescence and photochemistry: how, if at all, can different models of antenna organization be distinguished experimentally? Biochim Biophys Acta 1409:125–142
Black MT, Brearley TH, Horton P (1986) Heterogeneity in chloroplast photosystem II. Photosynth Res 8:193–207
Bolhar-Nordenkampf HR, Long SP, Baker NR, Öquist G, Schreiber U, Lechner EG (1989) Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Funct Ecol 3:497–514
Bowes JM, Crofts AR (1980) Binary oscillations in the rate of reoxidation of the primary acceptor of Photosystem II. Biochim Biophys Acta 590:373–384
Briantais JM, Vernotte C, Krause GH, Weiss E (1986) Chlorophyll a fluorescence of higher plants: chloroplasts and leaves. In: Govindjee Amesz J, Fork DC (eds) Light emission by plants and bacteria. Academic Press, Orlando, pp 539–583
Butler WL (1972) On the primary nature of fluorescence yield changes associated with photosynthesis. Proc Natl Acad Sci USA 69:3420–3422
Chylla RA, Garab G, Whitmarsh J (1987) Evidence for slow turnover in a fraction of photosystem II complexes in thylakoid membranes. Biochim Biophys Acta 894:562–571
Dau H (1994) Molecular mechanisms and quantitative models of variable photosystem II fluorescence. Photochem Photobiol 60:1–23
Duysens LNM, Sweers HE (1963) Mechanisms of the two photochemical reactions in algae as studied by means of fluorescence. In: Japanese Society of Plant Physiologists (ed) Studies on microalgae and photosynthetic bacteria. University of Tokyo Press, Tokyo, pp 353–372
Geacintov NE, Breton J (1987) Energy transfer and fluorescence mechanisms in photosynthetic membranes. CRC Crit Rev Plant Sci 5:1–44
Govindjee (1990) Photosystem II heterogeneity: the acceptor side. Photosynth Res 25:151–160
Govindjee (2004) Chlorophyll a fluorescence: a bit of basics and history. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 1–42
Govindjee, Papageorgiou G (1971) Chlorophyll fluorescence and photosynthesis: fluorescence transients. Photophysiology 6:1–50
Govindjee, Amesz J, Fork DC (eds) (1986) Light emission by plants and bacteria. Academic Press, Orlando, pp 539–583
Graan T, Ort DR (1986) Detection of oxygen-evolving photosystem II centers inactive in plastoquinone reduction. Biochim Biophys Acta 852:320–330
Groot ML, Pawlowicz NP, van der Wilderen LJGW, Breton J, van Stokkum IHM, van Grondelle R (2005) Initial electron donor and acceptor in isolated Photosystem II reaction centers identified with femtosecond mid-IR spectroscopy. Proc Natl Acad Sci USA 102:13087–13092
Haldimann P, Tsimilli-Michael M (2005) Non-photochemical quenching of chlorophyll a fluorescence by oxidized plastoquinone: new evidences based on modulation of the redox state of the endogenous plastoquinone pool in broken spinach chloroplasts. Biochim Biophys Acta 1706:239–249
Hiraki M, van Rensen JJS, Vredenberg WJ, Wakabayashi K (2003) Characterization of the alterations of the chlorophyll a fluorescence induction curve after addition of photosystem II inhibiting herbicides. Photosynth Res 78:35–46
Hiraki M, Vredenberg WJ, van Rensen JJS, Wakabayashi K (2004) A modified fluorometric method to quantify the concentration effect (pI50) of photosystem II-inhibiting herbicides. Pestic Biochem Physiol 80:183–191
Holzwarth AR, Müller MG, Reus M, Nowaczyk M, Sander J, Rögner M (2006) Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor. Proc Natl Acad Sci USA 103:6895–6900
Hsu BD, Lee J-Y (1995) Fluorescence quenching by plastoquinone in an oxygen-evolving photosystem-II-enriched preparation. J Photochem Photobiol 30:57–61
Joliot P, Joliot A (1964) Etude cinetique de la reaction photochimique liberant l’oxygene au cours de la photosynthèse. CR Acad Sci Paris 258:4622–4625
Joliot P, Joliot A (1973) Different types of quenching involved in photosystem II centers. Biochim Biophys Acta 305:302–316
Joliot P, Joliot A (1977) Evidence for a double hit process in photosystem II based on fluorescence studies. Biochim Biophys Acta 462:559–574
Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99(15):10209–10214
Klimov VV, Krasnovskii AA (1981) Participation of pheophytin in the primary processes of electron transfer at the reaction centers of photosystem II. Biophysics 27:186–198
Koblizek M, Kaftanm D, Nedbal L (2001) On the relationship between the non-photochemical quenching of the chlorophyll fluorescence and the photosystem II light harvesting efficiency. A repetitive flash fluorescence study. Photosynth Res 68:141–152
Kolber Z, Prasil O, Falkowski P (1998) Measurements of variable chlorophyll fluorescence using fast repetition rate technique. I. Defining methodology and experimental protocols. Biochim Biophys Acta 1367:88–106
Kramer DM, DiMarco G, Loreto F (1995) Contribution of plastoquinone quenching to saturation pulse-induced rise of chlorophyll fluorescence in leaves. In: Mathis P (ed) Photosynthesis: from light to biosphere, vol I. Kluwer Academic Publishers, Dordrecht, pp 147–150
Krause GH, Weiss E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Krause GH, Briantais JM, Vernotte C (1982) Photoinduced quenching of chlorophyll fluorescence in intact chloroplasts and algae: resolution into two components. Biochim Biophys Acta 679:116–124
Kurreck J, Schödel R, Renger G (2000) Investigation of the plastoquinone pool size and fluorescence quenching in thylakoid membranes and Photosystem II (PSII) membrane fragments. Photosynth Res 63:171–182
Lavergne J, Briantais J-M (1996) Photosystem II heterogeneity. In: Ort DR, Yocum CF (eds) Oxygenic photosynthesis: the light reactions. Series advances in photosynthesis. Kluwer Academic Publishers, Dordrecht
Lavergne J, Leci E (1993) Properties of inactive photosystem II centers. Photosynth Res 35:323–343
Lavergne J, Trissl HW (1995) Theory of fluorescence induction in photosystem II: Derivation of analytical expressions in a model including exciton-radical-pair equilibrium and restricted energy transfer between photosynthetic units. Biophys J 68:2474–2492
Lazár D (2006) The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct Plant Biology 33:9–30
Lazár D, Pospisil P (1999) Mathematical simulation of chlorophyll a fluorescence rise measured with 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea-treated barley leaves at room and high temperatures. Eur Biophys J 28:468–477
Lazár D, Tomek P, Ilík P, Nauš J (2001) Determination of the antenna heterogeneity of photosystem II by direct simultaneous fitting of several fluorescence rise curves measured with DCMU at different intensities. Photosynth Res 68:247–257
Mauzerall D (1972) Light induced fluorescence changes in Chlorella, and the primary photoreactions for the production of oxygen. Proc Natl Acad Sci USA 69:1358–1362
Melis A, Homann PH (1976) Heterogeneity of photochemical centers in system II of chloroplasts. Photochem Photobiol 23:343–350
Nedbal L, Trtilek M, Kaftan D (1999) Flash fluorescence induction: a novel method to study regulation of photosystem II. J Photochem Photobiol B Biol 48:154–157
Nedbal L, Soukupova J, Kaftan D, Whitmarsh J, Trtilek M (2000) Kinetic imaging of chlorophyll fluorescence using modulated light. Photosynth Res 66:3–12
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, 818 pp. ISBN 1-4020-3217-X
Rappaport F, Béal D, Joliot A, Joliot P (2007) On the advantages of using green light to study fluorescence yield changes in leaves. Biochim Biophys Acata 1767:56–65
Reifarth F, Christen G, Renger G (1997) Fluorometric equipment for monitoring P680+ reduction in PS II preparations and green leaves. Photosynth Res 51:231–241
Renger G, Eckert HJ, Bergmann A, Bernarding J, Liu B, Napiwotzki A, Reifarth F, Eichler HJ (1995) Fluorescence and spectroscopic studies of exciton trapping and electron transfer in photosystem II of higher plants. Aust J Plant Physiol 22:167–181
Schansker G, Toth SZ, Strasser RJ (2006) Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: the qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochimica et Biophysica Acta 1757:787–797
Schreiber U (1983) Chlorophyll fluorescence yield changes as a tool in plant physiology. The measuring system. Photosynth Res 4:361–373
Schreiber U (1986) Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth Res 9:261–272
Schreiber U, Krieger A (1996) Hypothesis: two fundamentally different types of variable chlorophyll fluorescence in vivo. FEBS Lett 397:131–135
Schreiber U, Neubauer C (1987) The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: II. Partial control by the photosystem II donor side and possible ways of interpretation. Z Naturforsch 42c:1255–1264
Schreiber U, Hormann H, Neubauer C, Klughammer C (1995) Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust J Plant Physiol 22:209–220
Shinkarev VP (2004) Photosystem II: oxygen evolution and chlorophyll a fluorescence induced by multiple flashes. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 197–229
Shinkarev VP, Xu C, Govindjee, Wraight CA (1997) Kinetics of the oxygen evolution step in plants determined from flash-induced chlorophyll a fluorescence. Photosynth Res 51:43–49
Steffen R (2003) Time-resolved spectroscopic investigation pf photosystem II. PhD thesis. Technical University Berlin
Steffen R, Christen G, Renger G (2001) Time-resolved monitoring of flash-induced changes of fluorescence quantum yield and decay of delayed light emission in oxygen-evolving photosynthetic organisms. Biochemistry 40:173–180
Steffen R, Eckert H-J, Kelly AA, Dörmann P, Renger G (2005) Investigations on the reaction pattern of photosystem II in leaves from Arabidopsis thaliana by time-resolved fluorometric analysis. Biochemistry 44(9):3124–3131
Stirbet AD, Govindjee, Strasser BJ, Strasser RJ (1998) Chlorophyll a fluorescence induction in higher plants: Modeling and numerical simulation. J Theor Biol 193:131–151
Strasser RJ (1978) The grouping model of plant photosynthesis. In: Akoyunoglou G (ed) Chloroplast development. Elsevier, North Holland, pp 513–524
Strasser RJ, Govindjee (1992) The F0 and the O-J-I-P fluorescence rise in higher plants and algae. In: Argyroudi-Akoyunoglou JH (ed) Regulation of chloroplast biogenesis. Plenum Press, New York, pp 423–426
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 321–362
Tomek P, Ilík P, Lazár D, Stroch M, Nauš J (2003) On the determination of QB-non-reducing photosystem II centers from chlorophyll a fluorescence induction. Plant Sci 164:665–670
Toth SZ, Schansker G, Strasser RJ (2005) In intact leaves, the maximum fluorescence level (F(M)) is independent of the redox state of the plastoquinone pool: a DCMU-inhibition study. Biochim Biophys Acta 708(2):275–282
Trissl H-W (2002) Theory of fluorescence induction: an introduction. http://www.biologie.uni-osnabrueck.de/biophysik/Trissl/teaching/teaching.html
Trissl H-W, Lavergne J (1995) Fluorescence induction from photosystem II: analytical equations for the yields of photochemistry and fluorescence derived from analysis of a model including exciton-radical pair equilibrium and restricted energy transfer between photosynthetic units. Aust J Plant Physiol 22:183–193
Trissl H-W, Gao Y, Wulf K (1993) Theoretical fluorescence induction curves derived from coupled differential equations describing the primary photochemistry of photosystem II by an exciton radical pair equilibrium. Biophys J 64:974–988
Vasil’ev S, Bruce D (1998) Nonphotochemical quenching of excitation energy in photosystem II. A picosecond time-resolved study of the low yield of chlorophyll a fluorescence induced by single-turnover flash in isolated spinach thylakoids. Biochemistry 37:11046–11054
Vermaas WFJ, Renger G, Dohnt G (1984) The reduction of the oxygen-evolving system in chloroplasts by thylakoid components. Biochim Biophys Acta 764:194–202
Vernotte C, Etienne AL, Briantais JM (1979) Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. Biochim Biophys Acta 545:519–527
Vredenberg WJ (2000) A three-state model for energy trapping and chlorophyll fluorescence in photosystem II incorporating radical pair recombination. Biophys J 79:25–38
Vredenberg WJ (2004) System analysis of photoelectrochemical control of chlorophyll fluorescence in terms of trapping models of Photosystem II: a challenging view. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 133–172
Vredenberg WJ, Duysens LNM (1963) Transfer and trapping of excitation energy from bacteriochlorophyll to a reaction center during bacterial photosynthesis. Nature 197:355–357
Vredenberg WJ, Rodrigues GC, van Rensen JJS (2002) A quantitative analysis of the chlorophyll fluorescence induction in terms of electron transfer rates at donor and acceptor sides of photosystem II. In: Proc. 12th Int. Congress Photosynthesis, Brisbane, 18–23 Aug., 2001 S14-10 on CD
Vredenberg WJ, van Rensen JJS, Rodrigues GC (2005) On the sub-maximal yield and photo-electric stimulation of chlorophyll a fluorescence in single turnover excitations in plant cells. Bioelectrochemistry 68:83–90
Vredenberg WJ, Kasalicky V, Durchan M, Prasil O (2006) The chlorophyll a fluorescence induction pattern in chloroplasts upon repetitive single turnover excitations: Accumulation and function of QB-nonreducing centers. Biochim Biophys Acta 1757:173–181
Vredenberg WJ, Durchan M, Prasil O (2007) On the chlorophyll fluorescence yield in chloroplasts upon excitation with twin turnover flashes (TTF) and high frequency flash trains. Photosynth Res 93:183–192. doi 10.1007/s11120-007-9150-8
Zhu X-G, Govindjee, Baker NR, deSturler E, Ort D, Long SP (2005) Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with photosystem II. Planta 223:114–133 doi 10.1007/s00425-005-0064-4
Acknowledgment
I thank Jack van Rensen for many discussions and Gustavo Rodrigues for assistance in doing the experiments.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendices
A. Derivation of the rFv(t) expression when light excitation rate k L for fluorescence emission is equal to the rate constant of the release of donor side quenching \(k_{s_1 } \)
Equation 3 gives the general expression for the normalized variable fluorescence rFv(t) = y 2(t) upon light excitation at a rate k L and under control of donor side quenching of which the release occurs with a rate constant \(k_{s_1 } \)
The equation is not applicable when \(k_{\text{L}} = k_{s_1 } .\) Here I give the derivation for the expression of y 2(t) for this particular condition. After rewriting Eq. 3 and series expansion of the function \( {\text{e}}^{ - (k_{s_1 } - k_{\text{L}} )t} \) (rows 2 and 3, respectively, in the derivation below) one obtains with substitution \(k_{\text{L}} = k_{s_1 } \) at the end of the third row:
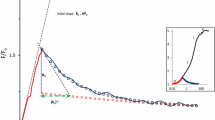
The plot of this relation is shown as the bold curve in Fig. 2a.
The relative variable fluorescence rFv in relation to the fraction q of (‘closed’) centers with Q −A for the particular case \(k_{\text{L}} = k_{s_1 } \) is easily obtained after substitution Eq. 4 in Eq. A.1. This gives
The plot of this rFv versus q relation for \(k_{\text{L}}=k_{s_1 } \) is shown as the bold curve in Fig. 2b.
B. Is the non-linear relation between rFv and the fractionq of centers with Q −A (‘closed’ RCs) (or between V and B, respectively, in Strasser’s terminology) a decisive indicator of energetic connectivity between RCs of PSII?
The answer is no, it is not. This will become clear from a closer look at the experimental procedure with which the fraction q (or B in Strasser’s terminology) of (closed) centers with Q −A is determined. B(t) is obtained, in the presence of DCMU, by numerical determination of the normalized area S(t) above the rFv(t) curve which gives the B(t) curve. The shape of the rFv(t) versus B(t) plot finally is used as a criterion for a nongrouping- (linear relation with rFv(t) = B(t)), or grouping- (hyperbolic relation between rFv(t) and B(t)) behavior of the PSII systems. In the latter case the connectivity) of the RCs of PSII is related to the empirically derived grouping parameter p by fitting the experimental rFv versus B relation with the hyperbolic relation
This relation (Strasser 1978), which is similar to one derived by Joliot and Joliot (1964), simplifies for C hyp = 0 (no grouping, or noncooperativity) to a linear relation rFv(t) = B(t). Equation B.1 is identical with Eq. 7 with B(t) = q(t). So far so good.
However, it should be realized that B(t) determined from the area above an experimental rFv curve gives the fraction q dsq of RCs in which the donor side quenching is released. As has been derived (see text and Eqs. 1 and 3) q dsq (= y 2) < q (= 1 − y 0). The unknown fraction y 1 of RCs with Q −A and rFv = 0 (due to quenching by donor side components) cannot be detected by the experimental area determination method; it remains hidden due to its quenched properties. Thus what in these graphic analyses routinely is considered as the rFv versus q relation in fact is the non-linear relation between rFv and q dsq fraction of RCs in which fluorescence quenching is released. Its non-linearity is quantitatively related to the release of donor side fluorescence quenching of which the rate constant becomes apparent as an approximately exponential rise in the tens of μs time range in ultra short STFs (Steffen 2003). Theoretically one would have found (see text) a linear relation between rFv and the fraction of closed centers if (i) the fraction q could have been estimated instead of q dsq and (ii) the effect of other inductors is comparatively small. In general the discrepancy between the outcome of the theoretical and experimental rFv versus q relation (with exclusion of improbable systematic errors in the experimental approach) might be caused by (impact factor is presumed to descend with order):
-
1.
Neglecting fluorescence quenching by redox intermediates at the donor side of PSII (donor side quenching).
-
2.
The fact that the closure of RCs in PSII is a double hit trapping process in which closure occurs via semi-open RCs (with 100% Q −A ) formed from open centers (100% Q A) in the first hit, as described in the Three State Trapping Model (TSTM).
-
3.
As yet unknown processes including that associated with (changes in) photo-electric fields.
-
4.
A variable and time dependent excitation rate k L caused for instance by intersystem energy transfer (connectivity) between PSUs of PSII.
-
5.
A combination of 1–4.
C. On the significance of the rFv versus complementary area (B) relation in the concept of the double hit trapping model (TSTM)
The normalized area B(t) above an experimental rFv curve measured in the presence of DCMU does not bear a simple relation to the fraction of closed PSII centers q(t) when the concept of TSTM is adopted. Here it is shown that, within this concept, the rFv versus B relation is non-linear, even under conditions at which k L is time independent (no connectivity) and the effect of donor side quenching is negligible, for instance at \(k_{\text{L}} \ll k_{s_1 } .\) In that case (see Hiraki et al. 2003; Vredenberg 2004 for illustration of scheme and meaning of subscript numbering) the reaction pattern can be represented by the scheme y 0 → y 2 → y 4 with rate constant k L for both steps; y 0 (=1), y 2 and y 4 refer to the open (y0), semi-open(-closed) and closed state of PSII systems with relative fluorescence yields rFv equal to 0, 0.5 and 1, respectively. In this simple form and assuming a time-independent excitation rate k L, the solution of the ODEs for y 0, y 2 and y 4 are identical to those given in Eqs. 1–3 with the proper substitutions of the subscripts for the y-states in Eqs. 2 and 3 and substituting \(k_{s_1 }=k_{\text{L}} {\text{.}}\) This gives (see also Eqs. 3a and A.1]), according to definitions:
and
Equations C.3 and C.4 show that rFv is non-linearly related to the area B above rFv under conditions in which donor side quenching and intersystem energy transfer can be excluded. Thus a double hit trapping mechanism like TSTM causes a non-linear relation between the relative variable fluorescence (rFv) and the area above the induction curve in the absence of donor side quenching and of connectivity between PSUs.
Rights and permissions
About this article
Cite this article
Vredenberg, W.J. Analysis of initial chlorophyll fluorescence induction kinetics in chloroplasts in terms of rate constants of donor side quenching release and electron trapping in photosystem II. Photosynth Res 96, 83–97 (2008). https://doi.org/10.1007/s11120-007-9287-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9287-5