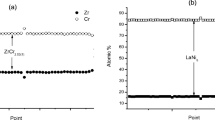
The ZrNi1.2Mn0.5Cr0.2V0.1 alloy was qualified using isotherms of hydrogen desorption in a gas atmosphere, equilibrium curves of hydrogen desorption in a 30% KOH solution, and X-ray diffraction data. The equilibrium pressure of the ZrNi1.2Mn0.5Cr0.2V0.1 alloy in a gas atmosphere at 20°C that was equal to ~1 atm (101,325 Pa) indicated that the alloy could be used in Ni–MH batteries, and the equilibrium hydrogen desorption curve obtained by the electrochemical method indicated that insignificant self-discharge could occur. The alloy samples crystallized at different cooling rates showed different quantitative phase composition. Electrodes produced from the sample with a greater amount of the Zr7Ni10 phase activated faster and those with a greater amount of the C15 and C14 phases had higher maximum discharge capacity. After the electrodes were exposed to air for 14 days, the Zr7Ni10 phase decreased by 7 vol.% and the total content of the C15 and C14 phases increased by 7 vol.% in the sample with a greater amount of the Zr7Ni10 phase (~ 24 vol.%). No changes in quantitative phase composition were found in the sample with a smaller amount of the Zr7Ni10 phase (~12 vol.%). Electrodes prepared from the sample with a lower content of the Zr7Ni10 phase showed better cyclic stability both before and after a pause in the cycles. The loss of the maximum achieved discharge capacity of these electrodes for 100 cycles with a pause in the cycles for 14 days (after the 80th cycle) and for 200 cycles with a pause in the cycles for 45 days (after the 150th cycle) was only 2 and 16%, and that of the electrodes with a higher content of the Zr7Ni10 phase was 30 and 52%. Thus, the electrodes produced from the alloy sample that showed stable quantitative phase composition according to X-ray diffraction had better hydrogenation–dehydrogenation cyclic stability when exposed to air in powder form for 14 days. Taking into account some similarities in hydrogenation-dehydrogenation and exposure to air of the zirconium alloys (oxidation of alloy components, significant increase in nickel surface concentration, etc.), the better cyclic stability of the electrodes was logically assumed to be due to more stable quantitative phase composition of the surface. The relationship between the electrochemical properties of the ZrNi1.2Mn0.5Cr0.2V0.1 alloy and the cooling rate in crystallization (quantitative phase composition) allows the development of materials with predicted functional properties.




Similar content being viewed by others
References
Yu.M. Solonin, L.L. Kolomiets, S.M. Solonin, and V.V. Skorokhod, “Development of hydrogenating powder alloys for the electrodes of alkali batteries. I. Principles of preparing alloys that reversibly absorb hydrogen,” Powder Metall. Met. Ceram., 42, No. 7–8, 372–378 (2003).
Is There Future for Lithium–Ion Batteries? [in Ukrainian], https://www.imena.ua/blog/li-ion-future.
Lithium–Ion Battery, https://en.wikipedia.org/wiki.
Yu.M. Solonin, O.Z. Galiy, K.O. Graivoronskaya, M.O. Krapyvka, A.V. Samelyuk, and T.A. Selinskaya, “The effect of quantitative phase composition and type of components on the electrochemical characteristics of the ZrNiMnCrV alloy,” Powder Metall. Met. Ceram., 60, No. 1–2, 105–111 (2021).
O.Z. Galiy, Effect of Chemical and Phase Composition and Surface State on the Electrochemical Properties of ZrMnNiCrMe (Me = V, Al) Alloys and Associated Electrodes [in Ukrainian], Author’s Abstract of PhD Thesis in Chemical Sciences, Inst. Probl. Materialoved. Nats. Akad. Nauk Ukrainy, Kyiv (2018), p. 20.
Yu.M. Solonin, O.Z. Galiy, A.V. Samelyuk, L.O. Romanova, and K.O. Graysvoronska, “Effect of stage-by-stage exposure of the Zr–Mn–Cr–Ni–V alloy to air on its cyclic stability,” Fiz. Khim. Tverd. Tela, 18, No. 3, 313–320 (2017).
Yu.M. Solonin, O.Z. Galiy, E.A. Graivoronska, and V.A. Lavrenko, “Electrochemical properties of ZrMnCrNiV alloy in long-term cycling after air oxidation,” Powder Metall. Met. Ceram., 56, No. 9–10, 567–572 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 61, Nos. 5–6 (545), pp. 139–146, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solonin, Y.M., Galiy, O., Karpets, M. et al. Electrochemical Properties of the ZrNiMnCrV Alloy Depending on Quantitative Phase Composition. Powder Metall Met Ceram 61, 370–376 (2022). https://doi.org/10.1007/s11106-022-00323-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-022-00323-8