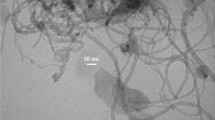
Materials that accumulate latent heat have become attractive to many aspects of human activity. However, their use is often limited by the issues of low thermal conductivity. Also, the transition from a solid to a molten state causes difficulties in storing materials, requiring special heat exchangers, which increases energy costs. These issues can be addressed by generating polydispersed powders for heat storage. Some shape-stable materials with a phase transition were obtained from melts by mixing halloysite nanotubes with carnauba wax. As a result, several solid samples of wax/nanotube were prepared by grinding at a 70/30, 60/40, and 50/50 mass ratio. Pure wax demonstrated the heat of the phase transition from solid to liquid state at 189.09 J/g. The latent heat of carnauba wax is approximately 25% greater than paraffin's. The created composite materials had a lower latent heat than wax, namely 99.39, 90.25, and 81.26 J/g for 70/30, 60/40, and 50/50 samples, respectively. Elementary mapping of nanomaterials revealed a uniform distribution of nanotubes in the waxy mass. According to X-ray diffraction analysis, the components did not form new crystallines during material preparation but manifested in the materials as physical mixtures. Also, the components did not chemically interact with each other when heated, which is a valuable property in the accumulation of thermal energy materials. It was found that the energy absorbed value and given heat by wax weighing 4 g, depending on weather conditions, was 26.96–242.60 J and 49.22–150.02 J.






Similar content being viewed by others
References
C. Lia, B. Xiea, J. Chena, Z. Heb, Z. Chenc, and Ya. Long, “Emerging mineral-coupled composite phase change materials for thermal energy storage,” Energy Conversion Management, 183, 633–644 (2019).
S. Song, T. Zhao, F. Qiu, W. Zhu, T. Chen, Y. Guo, Y. Zhang, Y. Wang, R. Feng, Y. Liu, C. Xiong, J. Zhou, and L. Dong, “Natural microtubule encapsulated phase change material with high thermal energy storage capacity,” Energy, 172, 1144–1150 (2019).
W. Wu, W. Wu, and S. Wang, “Form-stable and thermally induced flexible composite phase change material for thermal energy storage and thermal management applications,” Appl. Energy, 236, 10–21 (2019).
Sari, A. Karaipekli, and C. Alkan, “Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel formstable composite phase change material,” Chemical Eng. J., 155, 899–904 (2009).
S.Ya. Brychka and B.I. Bondarenko, “Maintaining stable nanodispersed cerium oxide for heat transfer processes,” Energy Technol. Resource Saving, No. 2, 36–42 (2020).
Anica Trp,”An experimental and numerical investigation of heat transfer during technical grade paraffin melting and solidification in a shell-and-tube latent thermal energy storage unit, Solar Energy, 79, Issue 6, December 2005, 648–660 (2005).
L. Bayés-García, L. Ventolà, R. Cordobilla, R. Benages, T. Calvet, and M.A. Cuevas-Diarte, “Phase change materials (PCMs) microcapsules with different shell compositions: preparation, characterization and thermal stability,” Solar Energy Mater. Solar Cells., 94, 1235–1240 (2010).
Q.H. Meng and J.L. Hu,” A poly (ethylene glycol)-based smart phase change material,” Solar Energy Mater. Solar Cells, 92, 1260–1268 (2008).
Y. Xia, H. Zhang, P. Huang, C. Huang, F. Xu, Y. Zou, H. Chu, E. Yan, and L. Sun, “Graphene-oxide-induced lamellar structures used to fabricate novel composite solid-solid phase change materials for thermal energy storage,” Chemical Eng. J., 362, 909–920 (2019).
Krup, G. Mikova, and A.S. Luyt, “Phase change materials based on low-density polyethylene/paraffin wax blends,” Europ. Polymer J., 43, 4695–4705 (2007).
Dincer and S. Dost, “A perspective on thermal energy storage system for solar energy storage applications,” Int. J. Energy Research,” 20, 547–557 (2002).
S. Song, F. Qiu, W. Zhu, Y. Guo, Y. Zhang, Y. Ju, R. Feng, Y. Liu, Z. Chen, J. Zhou, C. Xiong, and L. Dong, “Polyethylene glycol/halloysite@Ag nanocomposite PCM for thermal energy storage: Simultaneously high latent heat and enhanced thermal conductivity,” Solar Energy Mater. Solar Cells, 193, 237–245 (2019).
S.Ya. Brychka, Halloysite and Imogolite Nanotubes Chemistry [in Ukrainian], Vidavnichy dim "Kyi", Kyiv (2016). 258 pp.
H. Ji, D.P. Sellan, M.T. Pettes, X. Kong, J. Ji, and L. Shi, “Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage,” Energy Environ Sci., 7, 1186–1192 (2014).
S. Thanakkasaranee and J. Seo, ”Effect of halloysite nanotubes on shape stabilities of polyethylene glycol-based composite phase change materials International,” J. Heat Mass Transfer, 132 (2019), 154–161.
Y. Zhou, X. Wang, X. Liu, D. Sheng, F. Ji, L. Dong, S. Xu, H. Wu, and Y. Yang,” Polyurethane-based solid-solid phase change materials with halloysite nanotubes hybrid graphene aerogels for efficient light- and electro-thermal conversion and storage,” Carbon, 142, 558–566 (2019).
Kazuo Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th Edition, Wiley-Interscience (2009), 424 pp.
Acknowledgments
The work was performed with partial support of the Swedish SSF foundation
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Poroshkova Metallurgiya, Vol. 61, Nos. 5–6 (545), pp. 16–25, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Simeiko, K., Sydorenko, M. & Brichka, S.Y. Heat Accumulation by Wax Polydisperse Nanomaterials with Phase Transition. Powder Metall Met Ceram 61, 269–277 (2022). https://doi.org/10.1007/s11106-022-00314-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-022-00314-9