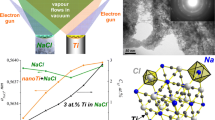
Chemically pure (ligand-free) 5–70 nm iron nanoparticles were synthesized by electron-beam physical vapor deposition (EB-PVD) in a porous NaCl matrix. The influence of chemical composition, substrate temperature, and isothermal treatment on the dimensional, structural, and phase composition of nanoparticles in the Fe–O system was studied. For this purpose, independent molecular fluxes of Fe and NaCl were applied to a fixed substrate by electron-beam physical vapor deposition (EB-PVD) at substrate temperatures of 45–400°C to produce 3–30 wt.% Fe–NaCl condensates. With increasing iron content, substrate temperature, and heat treatment temperature, the average size of particles in the Fe–O system became greater. To study the oxidation kinetics of iron nanoparticles, the condensates produced at a substrate temperature of 45°C were isothermally treated in air at 200–650°C. The condensates were examined by scanning electron microscopy, transmission electron microscopy, and X-ray diffraction, and aqueous solutions of the condensates by dynamic light scattering. Being of small size and thus having high adsorption capacity for air and moisture oxygen, iron exothermally oxidized in the condensate when the vacuum chamber was opened and the condensate was separated from the substrate. At different substrate temperatures and after heat treatment, the nanoparticles may contain pure iron and iron oxides Fe3O4 and Fe2O3. In the studied condensates, the α-Fe phase is present only when the iron content is more than 20 at.%. This is explained by the fact that not all nanoparticles (crystallites) have time to oxidize to the Fe3O4 phase with increase in their sizes. In addition, Fe3O4 nanoparticles additionally adsorb oxygen. The ratio between the atomic percentage of oxygen and iron depends on the amount of iron, decreases with increasing iron content of the condensate, and even exceeds the value for the stoichiometric composition of Fe2O3, being equal to 1.5. The study shows that the EB-PVD method is universal in the use of inorganic materials for the synthesis and retention of pure nanoparticles of metals and their oxides.











Similar content being viewed by others
References
J. Wallyn, N. Anton, and T.F. Vandamme, “Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications–a review,” Pharmaceutics, 11, No. 601, 1–29 (2019).
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L.V. Elst, and R.N. Muller, “Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications,” Chem. Rev., 108, No. 6, 2064–2110 (2008).
G.A. Silva, “Introduction to nanotechnology and its applications to medicine,” Surg. Neurol., 61, Issue 3, 216–220 (2004).
A.K. Gupta and M. Gupta, “Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications,” Biomaterials, 26, Issue 18, 3995–4021 (2005).
M. Banobre–López, A. Teijeiro, and J. Rivas, “Magnetic nanoparticle–based hyperthermia for cancer treatment. Reports of practical oncology and radiotherapy,” Rep. Pract. Oncol. Radiother., 18, 397–400 (2013).
K. O’Grady, and R.L. White, and P.J. Grundy, “Whither magnetic recording,” J. Magn. Magn. Mater., 177–181, 886–891 (1998).
A. Akbarzadeh, M. Samiei, and S. Davaran, “Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine,” Nanoscale Res. Lett., 7, No. 1, 144–156 (2012).
V.I. Nikolaev, A.M. Shipilin, and I.N. Zakharova, “On assessing the sizes of nanoparticles with the Mossbauer effect,” Fiz. Tverd. Tela, 43, No. 8, 1455–1457 (2001).
C.V. Thach, N.H. Hai, and N. Chau, “Size controlled magnetite nanoparticles and their drug loading ability,” J. Korean Phys. Soc., 52, No. 5, 1332–1335 (2008).
A.P. Shpak, P.P. Gorbik, V.F. Chekhun, L.G. Grechko, I.V. Dubrovin, A.L. Petranovskaya, L.Yu. Vergun, O.M. Korduban, and L.B. Lerman, “Nanocomposites for biomedical applications based on ultrafine magnetite,” in: Physical Chemistry of Nanomaterials and Supramolecular Structures [in Russian], Naukova Dumka, Kyiv (2007), Vol. 1, pp. 45–87.
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Elst, and R. Muller, “Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications,” Chem. Rev., 108, No. 6, 2064–2110 (2008).
A. Espinosa, R. Di Corato, J. Kolosnjaj–Tabi, P. Flaud, T. Pellegrino, and C. Wilhelm, “Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment,” ACS Nano, 10(2), 2436–2446 (2016).
M. Salimi, S. Sarkar, R. Saber, H. Delavari, A.M. Alizadeh, and H.T. Mulder, “Magnetic hyperthermia of breast cancer cells and MRI relaxometry with dendrimer–coated iron–oxide nanoparticles,” Cancer Nanotechnol., 9, No 1, 1–19 (2018).
L.V. Kovalenko and G.E. Folmanis, Biologically Active Iron Nanopowders [in Russian], Nauka, Moscow (2006), p. 124.
S. Laurent, D. Forge, and M. Port, “Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations and biological applications,” Chem. Rev., 108, 2064–2110 (2008).
N.V.S. Vallabani and S. Singh, “Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics,” 3 Biotech., 8(6), No. 279, 1–23 (2018).
K. Hola, Z. Markova, G. Zoppellaro, J. Tucek, and R. Zboril, “Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances,” Biotechnol Adv., 33(6), 1162–1176 (2015).
B. Sutens, T. Swusten, K. Zhong, J.K. Jochum, M.J. Van Bael, V. Erik, W. Brullot, M. Bloemen, and T. Verbiest, “Tunability of size and magnetic moment of iron oxide nanoparticles synthesized by forced hydrolysis,” Materials (Basel), 9, No 7, 4–10 (2016).
Q. Li, Ch.W. Kartikowati, Sh. Horie, T. Ogi, T. Iwaki, and K. Okuyama, “Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles,” Sci. Rep., 7, No. 9894, 1–7 (2017).
Q. Feng, Y. Liu, J. Huang, K. Chen, J. Huang, and K. Xiao, “Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings,” Sci. Rep., 8, No 1:2082, 1–13 (2018).
T. Vangijzegem, D. Stanicki, and S. Laurent, “Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics,” Expert Opin. Drug Delivery, 16, No. 1, 69–78 (2019).
H. Jung, B. Park, C. Lee, J. Cho, J. Suh, J. Park, Y. Kim, J. Kim, G. Cho, and H. Cho, “Dual MRI T1 and T2(*) contrast with size–controlled iron oxide nanoparticles,” Nanomedicine, 10, No. 8, 1679–1689 (2014).
S. Cassim, A. Giustini, A. Petryk, R. Strawbridge, and P. Hoopes, “Iron oxide hyperthermia and radiation cancer treatment,” Proc. SPIE Int. Soc. Opt. Eng., 7181, 1–8 (2009).
L. Kopanja, S. Kralj, D. Zunic, B. Loncar, and M. Tadić, “Core–shell superparamagnetic iron oxide nanoparticle (SPION) clusters: TEM micrograph analysis, particle design and shape analysis,” Ceram. Int., 42, No. 9, 10976–10984 (2016).
M. Tadić, S. Kralj, M. Jagodic, D. Hanzel, and D. Makovec, “Magnetic properties of novel superparamagnetic iron oxide nanoclusters and their peculiarity under annealing treatment,” Appl. Surf. Sci., 322, 255–264 (2014).
M. Tadić, V. Kusigerski, D. Marković, M. Panjan, I. Milošević, and V. Spasojević, “Highly crystalline superparamagnetic iron oxide nanoparticles (SPION) in a silica matrix,” J. Alloys Compd., 525, 28–33 (2012).
B.A. Movchan, “Electron-beam evaporation and physical vapor deposition of inorganic materials with amorphous, nanosized, and microsized structure,” in: Nanosystem, Nanomaterials, and Nanotechnology (Collected Scientific Papers) [in Russian], Akademperiodika, Kyiv (2004), Vol. 2, Issue 4, pp. 1103–1125.
I.S. Chekman, A.M. Serdyuk, Yu.I. Kundiev, I.M. Trakhtenberg, S.P. Kaplinskii, and V.F. Babiy, “Nanotoxicology: research areas,” Dovkil. Zdor., No. 7, 3–7 (2009).
L.A. Kulskii, Silver Water [in Russian], Osvita, Kyiv (1977), p. 176.
L.E. Foster, Nanotechnology: Science, Innovation, and Opportunity, Prentice Hall, USA (2005).
B.A. Movchan, “Inorganic materials and coatings produced by EBPVD,” Surf. Eng., 22, No. 1, 35–45 (2006).
B.A. Movchan, “Electron-beam nanotechnology and new materials in medicine—first steps,” Visn. Farmakol. Farm., No. 12, 5–13 (2007).
I.S. Chekman, B.A. Movchan, L.A. Krushinskaya, M.I. Zagorodny, Yu.A. Kurapov, and M.V. Kardash, “Nanosilver: production techniques, pharmacological properties, and indications,” Mystetsv. Likuv., No. 5(51), 32–34 (2008).
Yu.A. Kurapov, L.A. Krushinskaya, V.F. Gorchev, S.E. Litvin, and B.A. Movchan, “Analysis of colloidal systems based on Cu–O–H2O and Ag–O–H2O nanoparticles produced by molecular beam method,” Dop. Nats. Akad. Nauk Ukrainy, No. 7, 176–181 (2009).
V.E. Orel, P.P. Loshitskii, Yu.A. Kurapov, M.O. Nikolov, I.I. Dziatkovska, A.V. Ormanov, Yu.G. Melnik, and N.M. Dziatkovska, “Studying the effect of magnetically sensitive complex and inhomogeneous electromagnetic field on nonlinear dynamics of tumor growth and survival of animals with Guerin’s carcinoma. Electronics and connection,” 3rd Topical Issue Elektron. Nanotekhonol., 126–130 (2010).
B.A. Movchan, Yu.A. Kurapov, G.G. Didikin, S.G. Litvin, and S.M. Romanenko, “Control of the composition and structure of Fe–O nanoparticles during Fe3O4 electron beam evaporation,” Powder Metall. Met. Ceram., 50, No. 3–4, 167–172 (2011).
Y.A. Kurapov, E.M. Vazhnichaya, S.E. Litvin, S.M. Romanenko, G.G. Didikin, T.A. Devyatkina, Y.V. Mokliak, and E.I. Oranskaya, “Physical synthesis of iron oxide nanoparticles and their biological activity in vivo,” SN Appl. Sci., 1, No. 102 (2019).
Ukrainian Patent 92556, Method of Producing Metal–Oxygen Nanoparticles of Required Composition by Electron-Beam Evaporation and Condensation in Vacuum [in Ukrainian], publ. November 10 (2010), Bull. No. 21, p. 8.
Match! Phase Identification from Powder Diffraction, Version 1.9a, Crystal Impact 2003–2009.
A.D. Lebedev, Yu.N. Levchuk, A.V. Lomakin, and V.A. Noskin, Laser Correlation Spectroscopy and Biology [in Russian], Naukova Dumka, Kyiv (1987), p. 256.
A.D. Lebedev, A.V. Lomakin, and V.A. Noskin, “Laser correlation spectroscopy for biological objects in solutions,” in: Instrumented Methods in Physiology and Biophysics [in Russian], Nauka, Leningrad (1987), pp. 90–95.
E.G. Aftandilyants, K.G. Lopatko, Ya.V. Zaulicnhy, M.V. Karpets, and A.A. Shcheretskii, “Phase transformations in nanoparticles produced by electrospark deposition of metallic granules,” Elektr. Kont. Elektrod. Ser. Kompoz. Sloist. Grad. Mater. Koat., 112–128 (2014).
N.T. Gladkikh, A.P. Kryshtal, and S.I. Bogatyrenko, “Melting point of nanoparticles and vacancy formation energy,” Zh. Tekh. Fiz., 80, Issue 1, 111–114 (2010).
R.W. Cahn and P. Haasen, “Phase transformations in metals and alloys and alloys with special physical properties,” in: Physical Metallurgy [Russian translation], Metallurgiya, Moscow (1987), Vol. 2, p. 624.
Acknowledgements
The effort was funded from the budget Ukrainian program “Support in the Development of Priority Research Areas” (KPKVK 6541230).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 60, Nos. 7–8 (540), pp. 80–94, 2021.
Rights and permissions
About this article
Cite this article
Kurapov, Y., Lytvyn, S., Didikin, G. et al. Electron-Beam Physical Vapor Deposition of Iron Nanoparticles and their Thermal Stability in the Fe–O System. Powder Metall Met Ceram 60, 451–463 (2021). https://doi.org/10.1007/s11106-021-00256-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00256-8