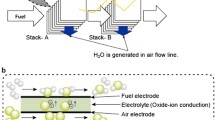
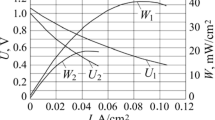
Fuel cells are today among the most efficient and environmentally friendly devices for the production of electricity. They are developing rapidly and are already in the early stages of commercial implementation. Solid oxide fuel cells (SOFCs) are largely promising as they show the highest efficiency and flexibility (H2, CnHm, CO, etc.) and do not need platinum group catalysts. Much attention is paid to improving the structure of SOFCs to increase their electrical properties. Different research groups commonly study the electrical properties of SOFCs under different conditions depending on the available equipment and the capabilities of their laboratories; this complicates an overall comparison between the electrical properties of fuel cells. In this regard, this research focuses on establishing the dependence of the specific electrical power (P0.7) at 0.7 V voltage for SOFCs on the hydrogen concentration in model fuel (a mixture of hydrogen and inert gas), other experimental conditions being equal. The specific electrical power P0.7 was chosen as one of the most important indicators for SOFC operation in power systems. The experimental dependence of P0.7 on the hydrogen concentration (CH2) in model fuel for different SOFCs was fitted with the least squares method to polynomial, logarithmic, and linear functions and the factor of coincidence (determination factor R2) with the experimental data was calculated. The quadratic and linear fitting functions were found to be most accurate among the functions considered and showed an average relative fitting error of no more than 9 and 12%, respectively. The prospects of the proposed hypothesis are that the fitted dependences of the SOFC electrical indicators can be used not only to predict the electrical properties of SOFCs in different operating conditions but also to facilitate the comparison of their electrical properties.






Similar content being viewed by others
References
S.C. Singhal and K. Kendall, High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications, Elsevier, Oxford, UK (2003), ISBN 13: 978–1–85617–387–2.
J. Molenda, K. Swierczek, and W. Zajac, “Functional materials for the IT-SOFC,” J. Power Sources, 73, 657–670 (2007).
Y.M. Brodnikovskyi, “Review: anode of solid oxide fuel cell,” Elektron. Mikrosk. Mitsn. Mater., Issue 19, 145–149 (2013).
V. Mokiychuk and N. Lysunenko, “Study of current–voltage characteristic of the solid oxide fuel cell,” Syst. Obrob. Inf., Issue 4, 45– 47 (2014).
Fuel Cell Handbook, 7th ed., EG&G Technical Services, Inc. (2004), p. 427.
Y.L. Leng, S.H. Chan, K.A. Khor, and S.P. Jiang, “Performance evaluation of anode–supported solid oxide fuel cells with thin film YSZ electrolyte,” Int. J. Hydrogen Energy, 29, 1025–1033 (2004).
Z. Wang, “Anode-supported SOFC with 1Ce10ScZr modified cathode/electrolyte interface,” J. Power Sources, 156, 306–310 (2006).
Y. Kim, T. Holme, T. Gür, and F. Prinz, “Surface-modified low-temperature solid oxide fuel cell,” Adv. Funct. Mater., 21, 4684–4690 (2011).
G. DiGiuseppe and L. Sun, “On the identification of impedance spectroscopy processes of an SOFC under different hydrogen concentration,” J. Fuel Cell Sci. Technol., 9, Issue 5, 051004–5 (2012).
L. Almar, B. Colldeforns, L. Yedra, S. Estrade, F. Peiró, A. Morata, T. Andreu, and A. Tarancon, “High-temperature long-term stable ordered mesoporous Ni–CGO as an anode for solid oxide fuel cells,” J. Mater. Chem. A, 1, 4531–4538 (2013).
Polishko, S. Ivanchenko, R. Horda, Ye. Brodnikovskyi, N. Lysunenko, and L. Kovalenko, “Tape casted SOFC based on Ukrainian 8YSZ powder,” Mater. Today: Proc., 6, No. 2, 236–241 (2019).
M. Rokni, “Addressing fuel recycling in solid oxide fuel cell systems fed by alternative fuels,” Energy, 137, 1013–1025 (2017).
E.T. Volodarskii, V.V. Kukharchuk, and V.O. Podzharenko, Metrological Support of Measurement and Instrumentation: Handbook [in Ukrainian], VELES, Vinnitsa (2001), p. 219.
G.K. Getman and S.L. Marikutsa, “Analysis of analytical functions for fitting of the universal magnetic characteristic of direct and intermittent current propulsion engines,” Visn. Dnipropetr. Nats. Univ. Zalizn. Trans., Issue 37, 63–71 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 60, Nos. 5–6 (539), pp. 118–128, 2021.
Rights and permissions
About this article
Cite this article
Lysunenko, N., Brodnikovskyi, Y., Mokiichuk, V. et al. The Influence of Hydrogen Concentration in an Ar–H2 Mixture on the Electrical Properties of Solid Oxide Fuel Cells. Powder Metall Met Ceram 60, 352–359 (2021). https://doi.org/10.1007/s11106-021-00245-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00245-x