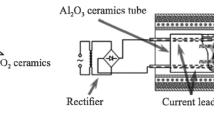
Zirconium dioxide is characterized by highly mobile anions at high temperatures and is thus capable of transmitting electrical current in these conditions, which can influence its interaction with metals. The effect of current passing through the interface between the zirconia ceramics and metal melts (copper, nickel, Cu–17.5 Ga, Ni–20 Cr) was studied. Current leads to the complete spreading of melts over the zirconia ceramic surface. Nickel and nickel–chromium melts spread faster, probably because these metals have higher melting points and, accordingly, experimental temperatures are greater. These results are explained by oxygen depletion of the ceramics near the contact with the metal melt as anions move to the electrode after connection of the positive current lead (cathode) to ZrO2. The oxygen deficiency can be considered to result from excess zirconium that dissolves in the melt, improving wetting. This was confirmed by microstructural studies, demonstrating significant dissolution of the zirconia ceramic cathode in the melt and formation of a thick transition zirconia layer on the anode. Since wetting was achieved, current was induced for brazing the ZrO2 ceramics to molybdenum. Dry joints were observed with copper used as a filler metal because the metal melt squeezed out of the brazing gap before wetting was achieved. In some cases, local overheating caused the ceramics to crack. Nickel and Ni–20 Cr fillers produced strong joints without dry areas. When Cu–45 Ni was used as a filler, the brazing gap was not filled in all samples, the ceramic cracked in local overheating in some samples, and other samples were quite strong. Zirconia ceramics and heat-resistant steel were brazed together with the Ni–20 Cr alloy. Current can thus be used in brazing ceramic materials.





Similar content being viewed by others
References
Yu.V. Naidich, “The wettability of solids by liquid metals,” Prog. Surf. Membr. Sci., 14, 353–487 (1981).
E. Saiz, R.M. Cannon, and A.P. Tomsia, “High-temperature wetting and the work of adhesion in metal–oxide systems,” Ann. Rev. Mat. Res., 38, 197–226 (2008).
C. Peden, K.B. Kidd, and N.D. Shinn, “Metal–metal-oxide interfaces: a surface science approach to the study of adhesion,” J. Vac. Sci. Technol., 9, 1518–1524 (1991).
Nogi Kiyoshi, Hiroyuki Takeda, and Kazumi Ogino, “Effect of applying a DC-voltage on the wettability of zirconia by liquid copper and on copper-zirconia joining,” Mater. Trans., 31, No. 1, 83–90 (1990).
Nogi Kiyoshi, Takeda Hiroyuki, and Ogino Kazumi, “Effect of applied DC voltage on the wettability of zirconia by liquid iron and strengthening of sprayed zirconia to iron,” ISIJ Int., 30, No. 12, 1092–1100 (1990).
Nogi Kiyoshi, Takeda Hiroyuki, Komatsu Masao, Iwamoto Nobuya, and Ogino Kazumi, “Effect of applying a DC voltage on the reaction at the Al–ZrO2 interface,” J. Jap. Inst. Met., 55, No 11, 1269–1273 (1991).
T. Yamazaki and A. Suzumura, “Monitoring of reactive metal’s diffusion and reaction at Ag–Cu–Ti/ZrO2 brazed interface,” Mater. Trans., JIM, 37, No. 5, 1103–1108 (1996).
Nan-nan Yang, Ping Shen, Bo Yang, Rui-fen Guo, and Qi-Chuan Jiang, “Significant improvement in the wettability of ZrO2 by molten Al under the application of a direct current,” Mater. Des., 111, 158–163 (2016).
Lian-Teng Yu, Ping Shen, Bo Yang, Rui-Fen Guo, Ni Zhang, and Qi-Chuan Jiang, “Polarity effects on the wettability and interfacial chemistry at Cu–YSZ interface by applying a direct current,” J. Eur. Ceram. Soc., 38, No. 4, 1790–1795 (2018).
Cu–Ni, Data from SGTE 2014 Alloy Database (Internet resource), http://www.crct.polymtl.ca/fact/phase_diagram.php?file=Cu-Ni.jpg&dir=SGTE2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 60, Nos. 5–6 (539), pp. 76–81, 2021.
Rights and permissions
About this article
Cite this article
Durov, O., Sydorenko, T. & Koval, O.Y. Brazing of ZrO2 Ceramics with Metallic Fillers Using Electrical Current. Powder Metall Met Ceram 60, 318–322 (2021). https://doi.org/10.1007/s11106-021-00242-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00242-0