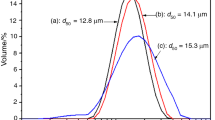
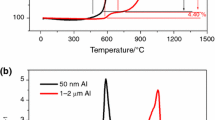
Commercially available aluminum PA-4 and ASD-1 powders were mechanochemically activated by Ga–In–Sn or Ga–In–Sn–Zn eutectic alloys (5 wt.%) and graphite (1–3 wt.%) in a mixer type ball mill. Subsequently, they were pressed (P = 4 MPa) into the pellets used to generate hydrogen from water via the hydrolysis process. X-ray diffraction study of the milled PA-4 powder revealed the presence of four phases, including aluminum, graphite, and two In–Sn intermetallic compounds (In3Sn and In1–xSnx, were x ≈ 0.04). SEM morphological characterization of the surfaces of the prepared pellets showed the presence of clear interfaces between the grains of aluminum. The quantitative analysis by EDX showed a uniform distribution of the activating additives over the pellet surface, where the graphite was also partly aggregated. Studies on the hydrolysis kinetics of Al-based pellets demonstrated that it readily proceeds at temperatures of ≥5°C. At the same time, the efficiency of hydrogen generation depends on the amount of the added graphite, particle size of aluminum powders, duration of their mechanochemical treatment, and the hydrolysis temperature. The introduction of graphite and eutectic alloys into the aluminum powders during their mechanochemical treatment significantly accelerate the hydrolysis process. Besides, the process is faster, the more graphite is added. The obtained values of the hydrogen evolution rate and hydrogen yield during the hydrolysis of pellets from finer ASD-1 powder exhibit significantly superior performance compared to pellets’ hydrolysis from coarser PA-4 powder. Increased duration of aluminum powders mechanochemical treatment from 1 to 4 h results in a significant increase in the hydrolysis rate of the prepared pellets and hydrogen yield. The rise of hydrolysis temperature from 5 to 25°C lead to an increase in the hydrolysis rate during its active stage.










Similar content being viewed by others
References
L.H. Kozin, S.V. Volkov, and I.N. Skryptun, Modern Hydrogen Energetics and Ecology, Akademperiodyka, Kyiv (2019), p. 364; DOI: https://doi.org/10.15407/akademperiodyka.392.364.
I.L. Varshavskyi, Energy-Accumulating Substances and Their Applications [in Russian], Naukova Dumka, Kyiv (1980), p. 240.
F.D. Manilevich, Yu.K. Pirskyy, B.I. Danil’tsev, A.V. Kutsyi, and V.A. Yartys, “Studies of the hydrolysis of aluminum activated by additions of Ga–In–Sn eutectic alloy, bismuth or antimony,” Mater. Sci., 55, No. 4, 536–547 (2020), DOI: https://doi.org/10.1007/s11003-020-00336-x.
E.I. Shkolnikov, A.Z. Zhuk, and M.S. Vlaskin, “Aluminum as energy carrier: Feasibility analysis and current technologies overview,” Renew. Sustain. Energy Reviews, 15, No. 9, 4611–4623 (2011), DOI: https://doi.org/10.1016/j.rser.2011.07.091.
E. Shkolnikov, M. Vlaskin, A. Iljukhin, A. Zhuk, and A. Sheindlin, “2W power source based on air-hydrogen polymer electrolyte membrane fuel cells and water-aluminum hydrogen macro-generator,” J. Power Sources, 185, No. 2, 967–972 (2008), DOI: https://doi.org/10.1016/j.jpowsour.2008.09.062.
H.Z. Wang, D.Y.C. Leung, M.K.H. Leung, and M. Ni, “A review on hydrogen production using aluminum and aluminum alloys,” Renew. Sustain. Energy Reviews, 13, No. 4, 845–853 (2009), DOI: https://doi.org/10.1016/j.rser.2008.02.009.
A.V. Parmuzina and O.V. Kravchenko, “Activation of aluminum metal to evolve hydrogen from water,” Int. J. Hydrogen Energy. 33, No. 12, 3073–3076 (2008); https://doi.org/10.1016/j.ijhydene.2008.02.025.
A. Babak and M. Korosh, “A novel method for generating hydrogen by hydrolysis of highly activated aluminum nanoparticles in pure water,” Int. J. Hydrogen Energy. 34, No. 19, 7934–7938 (2009).
V. Rosenband and A. Gany, “Application of activated aluminum powder for generation of hydrogen from water,” Int. J. Hydrogen Energy, 35, No. 20, 10898–10904 (2010), DOI: https://doi.org/10.1016/j.ijhydene.2010.07.019.
A.V. Ilyukhina, O.V. Kravchenko, B.M. Bulychev, and E.I. Shkolnikov, “Mechanochemical activation of aluminum with gallams for hydrogen evolution from water,” Int. J. Hydrogen Energy, 35, No. 5, 1905–1910 (2010), DOI: https://doi.org/10.1016/j.ijhydene.2009.12.118.
R.S. Nazarov, S.D. Kushch, O.V. Kravchenko, É.É. Fokina, and B.P. Tarasov, “Hydrogen-generating materials for the sources of hydrogen of the hydrolysis type,” Alternative Energetics and Ecology, 86, No. 6, 26–32 (2010).
M. Korosh and A. Babak, “Enhancement of hydrogen generation rate in reaction of aluminum with water. Int. J. Hydrogen Energy, 35, No. 11, 5227–5232 (2010); DOI: https://doi.org/10.1016/j.ijhydene.2010.03.016.
E. Czech and T. Troczynski, “Hydrogen generation through massive corrosion of deformed aluminum in water,” Int. J. Hydrogen Energy, 35, No. 3, 1029–1037 (2010), DOI: https://doi.org/10.1016/j.ijhydene.2009.11.085.
M.-Q. Fan, D.-Sh. Mei, D. Chen, Ch.-J. Lv, and K.-Y. Shu, “Portable hydrogen generation from activated Al–Li–Bi alloys in water,” Renewable Energy, 36, No. 11, 3061–3067 (2011), DOI: https://doi.org/10.1016/j.renene.2011.03.029.
Sh. Liu, M.-Q. Fan, Ch. Wang, Y.-X. Huang, D. Chen, L.-Q. Bai, and K.-Y. Shu, “Hydrogen generation by hydrolysis of Al–Li–Bi–NaCl mixture with pure water,” Int. J. Hydrogen Energy, 37, No. 1, 1014–1020 (2012), DOI: https://doi.org/10.1016/j.ijhydene.2011.03.029.
W.-Zh. Gai, W.-H. Liu, Zh.-Y. Deng, and J.-G. Zhou, “Reaction of Al powder with water for hydrogen generation under ambient condition,” Int. J. Hydrogen Energy, 37, No. 17, 13132–13140 (2012), DOI: https://doi.org/10.1016/j.ijhydene.2012.04.025.
Y. Yavor, S. Goroshin, J.M. Bergthorson, D.L. Frost, R. Stowe, and S. Ringuette, “Enhanced hydrogen generation from aluminum-water reactions,” Int. J. Hydrogen Energy, 38, No. 35, 14992–15002 (2013), DOI: https://doi.org/10.1016/j.ijhydene.2013.09.070.
H. Wang, Y. Chang, Sh. Dong, Zh. Lei, Q. Zhu, and P. Luo, “Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism,” Int. J. Hydrogen Energy, 38, No. 3, 1236–1243 (2013), DOI: https://doi.org/10.1016/j.ijhydene.2012.11.034.
C.C. Wang, Y.C. Chou, and C.Y. Yen, “Hydrogen generation from aluminum and aluminum alloys powder,” Procedia Eng., 36, 105–113 (2012), DOI: https://doi.org/10.1016/j.proeng.2012.03.017.
S. Prabu, Sh.-Ch. Hsu, J.-S. Lin, and H.-W. Wang, “Rapid hydrogen generation from the reaction of aluminum powders and water using synthesized aluminum hydroxide catalysts,” Topic in Catalysis, 2018. Online, DOI: https://doi.org/10.1007/s11244-018-0970-x.
X. Huang, T. Gao, X. Pan, D. Wei, C. Lv, L. Qin, and Y. Huang, “A review: Feasibility of hydrogen generation from the reaction between aluminum and water for fuel cell applications,” J. Power Sources, 229, 133–140 (2013), DOI: https://doi.org/10.1016/j.jpowsour.2012.12.016.
O.V. Kravchenko, K.N. Semenenko, B.M. Bulychev, and K.B. Kalmykov, “Activation of aluminum metal and its reaction with water,” J. Alloys Compd., 397, No. 1–2, 58–62 (2005), DOI: https://doi.org/10.1016/j.jallcom.2004.11.065.
S. Hu, X. Zhao, and J. Liu, “Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature,” Renew. Sustain. Energy Reviews, 92, 17–37 (2018), DOI: https://doi.org/10.1016/j.rser.2018.04.052
J.T. Ziebarth, J.M. Woodall, R.A. Kramer, and G. Choi, “Liquid phase-enabled reaction of Al–Ga and Al–Ga–In–Sn alloys with water,” Int. J. Hydrogen Energy, 36, No. 9, 5271–5279 (2011), DOI: https://doi.org/10.1016/j.ijhydene.2011.01.127.
W. Wang, D.M. Chen, and K. Yang, “Investigation on microstructure and hydrogen generation performance of Al-rich alloys,” Int. J. Hydrogen Energy, 35, No. 21, 12011–12019 (2010), DOI: https://doi.org/10.1016/j.ijhydene.2010.08.089.
W. Wang, X.M. Zhao, D.M. Chen, and K. Yang, “Insight into the reactivity of Al–Ga–In–Sn alloy with water,” Int. J. Hydrogen Energy, 37, No. 3, 2187–2194 (2012), DOI: https://doi.org/10.1016/j.ijhydene.2011.10.058.
W. Wang, W. Chen, X.M. Zhao, D.M. Chen, and K. Yang, “Effect of composition on the reactivity of Al-rich alloys with water,” Int. J. Hydrogen Energy, 37, No. 24, 18672–18678 (2012), DOI: https://doi.org/10.1016/j.ijhydene.2012.09.164.
M.Q. Fan, F. Xu, and L.X. Sun, “Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water,” Int. J. Hydrogen Energy, 32, No. 14, 2809–2815 (2007), DOI: https://doi.org/10.1016/j.ijhydene.2006.12.020.
T. He, W. Wang, W. Chen, W. Chen, D. Chen, and K. Yang, “Reactivity of Al-rich alloys with water promoted by liquid Al grain boundary phases,” J. Mat. Sci. Technol., 33, No. 4, 397–403 (2017), DOI: https://doi.org/10.1016/j.jmst.2016.11.013.
T. Huang, Q. Gao, D. Liu, S. Xu, C. Guo, J. Zou, and C. Wie, “Preparation of Al–Ga–In–Sn–Bi quinary alloy and its hydrogen production via water splitting,” Int. J. Hydrogen Energy, 40, No. 5, 2354–2362 (2015), DOI: https://doi.org/10.1016/j.ijhydene.2014.12.034.
B.D. Du, W. Wang, W. Chen, D.M. Chen, and K. Yang, “Grain refinement and Al-water reactivity of Al–Ga–In–Sn alloys,” Int. J. Hydrogen Energy, 42, No. 34, 21586–21596 (2017), https://doi.org/10.1016/j.ijhydene.2017.07.105.
Yu Y., Wang S., Wang X., Wang Q., Liu J. Semisolid Al–Ga composites fabricated at room temperature for hydrogen generation. RSC Adv. 2020. Vol. 10, No. 17. P. 10076–10081, DOI: https://doi.org/10.1039/c9ra10906d.
Zhou D.D., Fan J. Using grain refiner Al–3Ti–0.3C to improve Al-water reaction rate and yield. Mater. Trans. 2020. Vol. 61, No. 6. P. 1164–1171. DOI: https://doi.org/10.2320/matertrans.MT-MK2019005.
Streletskii A.N., Kolbanev I.V., Borunova A.B., Butyagin P.Yu. Mechanochemically activated aluminum: Preparation, structure, and chemical properties. J. Mater. Sci. 2004. Vol. 29, No. 16–17. P. 5175–5179, DOI: https://doi.org/10.1023/B:JMSC.0000039205.46608.1a.
X.N. Huang, C.J. Lv, Y. Wang, H.Y. Shen, D. Chen, and Y.X. Huang, “Hydrogen generation from hydrolysis of aluminum/graphite composites with a core-shell structure,” Int. J. Hydrogen Energy, 37, No. 9, 7457–7463 (2012), DOI: https://doi.org/10.1016/j.ijhydene.2012.01.126.
Yu. Plevachuk, V. Sklyarchuk, Sv. Eckert, G. Gerbeth, and R. Novakovic, “Thermophysical poperties of the liquid Ga−In−Sn eutectic alloy,” J. Chem. Eng. Data, 59, No. 3, 757–763 (2014), DOI: https://doi.org/10.1021/je400882q.
Yu.N. Grin, and R.E. Gladyshevskij, Gallids [in Russian], Metallurgiya Moscow (1989), p. 304.
H.M. Rietveld, “A profile refinement method for nuclear and magnetic structures,” J. Appl. Crystallogr., 2, No. 2, 65−71 (1969), DOI: https://doi.org/10.1107/S0021889869006558.
J. Rodriguez-Carvajal, “Recent advances in magnetic structure determination by neutron powder diffraction,” Physica B: Condensed Matter., 192, No. 1–2, 55–69 (1993), DOI: https://doi.org/10.1016/0921-4526(93)90108-I.
Acknowledgment
This work is supported by the NATO Programme “Science for Peace and Security” under grant G5233 “Portable Energy Supply”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Poroshkova Metallurgiya, Vol. 60, Nos. 5–6 (539), pp. 14–24, 2021.
Rights and permissions
About this article
Cite this article
Manilevich, F., Pirskyy, Y.K., Kutsyi, A. et al. Studies of Mechanochemically Activated Aluminum Powders for Generating Hydrogen from Water. Powder Metall Met Ceram 60, 268–277 (2021). https://doi.org/10.1007/s11106-021-00237-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00237-x