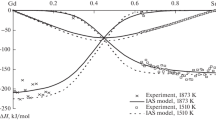
The calorimetry method was employed to determine the mixing enthalpies of Gd–Sn melts and the ideal associated solution (IAS) model to calculate and optimize the thermodynamic properties of Gd–Sn alloys at 1510, 1640, and 1873 K in the composition range 0 ≤ xSn ≤ 1.0. The minimum mixing enthalpies were –69.7 ± 0.6 kJ/mole (1873 K) and –77.9 ± 0.7 (1510 K) kJ/mole at xSn = = 0.45. Using our own and published data on the thermochemical properties of melts and compounds and assuming the formation of two associates in the melt, Gd2Sn and GdSn, we calculated the activities of components, enthalpies, Gibbs energies, and entropies of formation for the liquid alloys and intermediate phases within the IAS model. The thermodynamic activities of components in the studied melts showed very large negative deviations from the ideal solutions. The mixing enthalpies of Sn–Gd melts optimized within the IAS model agreed well with the experimental values. The \( \Delta {\overline{H}}_{\mathrm{Gd}}^{\infty } \) temperature dependence agrees only qualitatively with other experimental data because of great errors in the published data. The excess Gibbs energy and mixing enthalpy of Gd–Sn melts calculated with the IAS model at 1873 K greatly differed in magnitude, being indicative of a significant contribution of the entropy component to the excess molar Gibbs energy. According to the calculations, the minimum excess mixing entropy for Gd–Sn melts at 1873 K was –20.3 J/(mole · K) at xSn = 0.45. The calculated and optimized enthalpies and entropies of formation for intermetallic phases in the Gd–Sn system, along with the IAS model parameters for melts, were used to calculate the liquidus and solidus curves of the phase diagram. Good agreement with most experimental data on the phase equilibria involving liquid and crystalline phases was shown.






Similar content being viewed by others
References
V.N. Eremenko, M.V. Bulanova, and P.S. Martsenyuk, “Phase diagrams of binary systems of rare earth metals with tin,” in: Phase Equilibria, Structures, and Properties of Alloys [in Russian], Naukova Dumka, Kyiv (1990), pp. 70–100.
N.G. Kulagina, A.P. Bayanov, and N.M. Kulagin, “Study of Sn–REM (La, Nd, Gd, Er, Lu) binary systems,” Izv. Akad. Nauk SSSR. Met., No. 3, 211–216 (1985).
A. Palenzona, “Dynamic differential calorimetry of intermetallic compounds. 1. Heats of formation, heat and entropy of fusion of rare earth–tin compounds,” Thermochim. Acta, 5, No. 4, 473–480 (1973).
A. Percheron-Guegan, J.C. Achard, A. Bacha, C. Chatillon, and J.C. Mathieu, “Calorimetric study of some rare earth metals and their LnSn3 compounds,” in: C.J. Kevane and T. Moeller (eds.), Proc. 10th Rare Earth Res. Conf., Springfield (1973), pp. 1046–1051.
L. Liu and J. Zheng, “Phase diagram of the alloys in Gd–Sn binary system,” Acta Phys. Sin., 33, No. 8, 1155–1159 (1984).
A. Palenzona and S. Cirafici, “The Gd–Sn (gadolinium–tin) system,” J. Phase Equilib., 12, No. 6, 690–695 (1991).
M.V. Bulanova and P.S. Martsenyuk, “Prediction of the temperatures of certain nonvariant equilibria in rare-earth metal-group IV p-element systems,” Powder Metall. Met. Ceram., 30, No. 5, 424–427 (1991).
R.V. Skolozdra, A.G. Akselrud, V.K. Pecharskii, and O.E. Koretskaya, “New compounds in the Gd–Sn system and their crystalline structure,” Dokl. Akad. Nauk USSR. Ser. B, No. 12, 51–54 (1986).
N.G. Kulagina and A.P. Bayanov, “Thermodynamic properties of GdSn3 and is solutions in liquid tin,” Zh. Fiz. Khim., 48, No. 1, 233 (1974).
N.G. Kulagina, Thermodynamics of Interaction between Rare Earth Metals and Tin [in Russian], Author’s Abstract of PhD Thesis in Chemical Sciences, Sverdlovsk (1980), p. 21.
C. Colinet, A. Pasturel, A. Percheron-Guegan, and J. C. Achard, “Experimental and calculated enthalpies of formation of rare earth–tin alloys,” J. Less-Common Met., 102, No. 2, 167–177 (1984).
F.R. DeBoer, R. Boom, W.C.M. Mattens, A.R. Miedema, and A.K. Niessen, Cohesion in Metals. Transition Metal Alloys, Elsevier, Amsterdam (1989), p. 789.
A. Bacha, C. Chatillon-Colinet, A. Percheron, and L.-C. Mathieu, “Calorimetric measurements of the heat of dissolving gadolinium in tin. Determining the enthalpy of formation for compound GdSn3,” C.R. Acad. Sci., C274, No. 7, 680–683 (1972).
C. Colinet, “The thermodynamic properties of rare earth metallic systems,” J. Alloys Compd., 225, 409–422 (1995).
S.V. Meschel and O.L. Kleppa, “Standard enthalpies of formation of some rare earth stannides by high temperature direct synthesis calorimetry,” J. Alloys Compd., 238, 180–186 (1996).
A.K. Niessen, F.R. deBoer, R. Boom, P.F. DeChatel, W.C.M. Mattens, and A.R. Miedema, “Model predictions for the enthalpy of formation of transition metal alloys,” Calphad, 7, 51–70 (1983).
F.R. DeBoer, R. Boom, W.C.M. Mattens, A.R. Miedema, and A.K. Niessen, Cohesion in Metals, Transition Metal Alloys, North-Holland, Amsterdam (1988), p. 758.
V.I. Kober, I.F. Nichkov, and S.P. Raspopin, “Thermodynamic properties of liquid alloys of light REMs with fusible metals,” in: Proc. Sci. Assoc. Fifth All-Union Conf. Constitution and Properties of Metallic and Slag Melts. Part 2. Experimental Studies of Liquid and Amorphous Metals [in Russian], UNTs AN SSSR, Sverdlovsk (1983), pp. 334–336.
V.I. Kober, I.F. Nichkov, S.P. Raspopin, and V.I. Kuzminykh, “Thermodynamic properties of gadolinium alloys with fusible metals,” in: Alloys of Liquid Metals with Special Physical Properties. Rare Earth and Noble Metals [in Russian], Nauka, Moscow (1983), pp. 132–135.
F. Sommer, J. Schott, and H.-G. Krull, “Temperature dependence of partial and integral enthalpies of mixing of liquid rare-earth–Sn alloys,” J. Less-Common Met., 144, No. 1, 53–63 (1988).
J. Kim and Jung In-Ho, “Critical evaluation and thermodynamic optimization of the Sn–RE systems. Part II. Sn–RE system (RE = Gd, Tb, Dy, Ho, Er, Tm, Lu and Y),” Calphad, 55, Part 2, 134–156 (2016).
M.A. Shevchenko, M.I. Ivanov, V.V. Berezutskii, V.G. Kudin, and V.S. Sudavtsova, “Thermodynamic properties of Mn–Sc (REM) melts,” Zh. Fiz. Khim., 86, No. 12, 1914–1919 (2012).
A.T. Dinsdale, “SGTE data for pure elements,” Calphad, 15(4), 319–427 (1991).
I. Prigogine and R. Defay, Chemical Thermodynamics, Longmans, London (1954).
A.G. Morachevskii, A. Mokrievich, and E. Mayorova, “Analyzing the behavior of the excessive stability function using the ideal associated solution model,” Zh. Obsch. Khim., 59, No. 6, 1209–1214 (1989).
M.A. Turchanin, I.V. Belokonenko, and P.G. Agraval, “On applying the theory of ideal associated solution to describe the temperature–composition dependence for thermodynamic properties of binary alloys,” Rasplavy, No. 1, 58–69 (2001).
M.A. Shevchenko, V.G. Kudin, and V.S. Sudavtsova, “Correctness of thermodynamic properties of binary alloys calculated with the ideal associated solution model,” in: Current Issues of Physical Materials Science [in Russian], Proc. Inst. Probl. Materialoved. NAN Ukrainy, Kyiv (2012), Issue 21, pp. 67–74.
M.I. Ivanov, V.V. Berezutskii, M.O. Shevchenko, V.G. Kudin, and V.S. Sudavtsova, “Interaction in alloys of the europium-containing systems,” Dop. Nats. Akad. Nauk Ukrainy, No. 8, 90–99 (2013).
V.G. Kudin, M.A. Shevchenko, I.V. Mateiko, and V.S. Sudavtsova, “Thermodynamic properties of Al–La melts,” Zh. Fiz. Khim., 87, No. 3, 364–370 (2013).
V.S. Sudavtsova, M.I. Ivanov, V.V. Berezutskii, V.G. Kudin, and M.A. Shevchenko, “Mixing enthalpy for Al–Yb melts,” Zh. Fiz. Khim., 86, No. 8, 1311–1315 (2012).
M.A. Shevchenko, V.G. Kudin, N.G. Kobylinskaya, and V.S. Sudavstsova, “Thermodynamic properties and phase diagram of the Ce–Si system,” Ukr. Khim. Zh., 78, No. 6, 96–102 (2012).
M.O. Shevchenko, V.V. Berezutski, M.I. Ivanov, V.G. Kudin, and V.S. Sudavtsova, “Thermodynamic properties of alloys of the binary Al–Sm, Sm–Sn and ternary Al–Sm–Sn systems,” J. Phase Equilib. Diff., 36, No. 1, 39–52 (2015).
M.O. Shevchenko, M.I. Ivanov, V.V. Berezutski, and V.S. Sudavtsova, “Thermodynamic properties of alloys in the binary Ca–Ge system,” J. Phase Equilib. Diff., 36, No. 6, 554–572 (2015).
V.S. Sudavtsova, M.O. Shevchenko, M.I. Ivanov, V.G. Kudin, and N.V. Podoprigora, “Thermodynamic properties and phase equilibria of Nd–Ni alloys,” Powder Metall. Met. Ceram., 58, No. 9–10, 581–590 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 60, Nos. 3–4 (538), pp. 121–135, 2021.
Rights and permissions
About this article
Cite this article
Sudavtsova, V.S., Shevchenko, M.O., Kudin, V.G. et al. Thermodynamic Properties and Phase Equilibria in Gd–Sn Alloys. Powder Metall Met Ceram 60, 225–236 (2021). https://doi.org/10.1007/s11106-021-00231-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00231-3