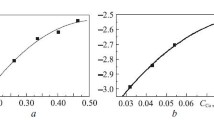
The solubility of molybdenum in Fe–Cu and Fe–Sn melts and the growth kinetics of Mo6Fe7 layer at the molybdenum–melt interface at 1200°C are examined. The solubility of molybdenum in these melts is well described by the following equations: lgCMo = (–3.957 ± 0.176) + (25.77 ± 7.94) XFe –– (143.02 ± 77.92) XFe 2 (Fe–Cu melt) and lgCMo = (–2.783 ± 0.011) + (7.563 ± 0.111) XFe –– (10.844 ± 0.245) XFe 2 (Fe–Sn melt). The melt composition (atomic fractions) in the three-phase Mo–Mo6Fe7–melt equilibrium is established: 6.4 · 10–3 Fe, 1.64 · 10–4 Mo, and Cu being the rest (Fe–Cu melt); 0.046 Fe, 3.48 · 10–3 Mo, and Sn being the rest (Fe–Sn melt). The substantial difference between the growth rate constants in the melts, kFe–Sn >> kFe–Cu, at the same iron activity a Fe is attributed to the effect of admixtures (Cu and Sn) on the growth of the Mo6Fe7 layer. Data on the solubility of molybdenum in Fe–Cu and Fe–Sn melts and the growth kinetics of Mo6Fe7 in these melts are obtained for the first time.







Similar content being viewed by others
References
C. Agte and J. Vacek, Tungsten and Molybdenum, NASA, Office of Scientific and Technical Information, Washington (1963).
Sintered Materials for Electrical Engineering and Electronics: Handbook [in Russian], Metallurgiya, Moscow (1981), p. 342.
O. K. Teodorovich, Yu. A. Isakov, R. V. Minakova, et al., “Effect of inhomogeneous structure of tungsten–copper contacts on their wear resistance,” in: Electrical Contacts and Electrodes [in Russian], Naukova Dumka, Kyiv (1977), pp. 24–25.
V. V. Skorokhod, V. P. Titov, M. E. Golovkova, and N. I. Filippov, “Interaction of molybdenum with cobalt–tin and cobalt–copper melts,” Powder Metall. Met. Ceram., 54, No. 1–2, 93–100 (2015).
T. B. Massalski, Binary Alloy Phase Diagrams, ASM, Metals Park, Ohio (1986), Vol. 2, p. 1124.
N. P. Lyakishev (ed.), Phase Diagrams of Binary Metallic Systems [in Russian], Mashinostroenie, Moscow (1997), Vol. 2, p. 1023.
G. Kostakis, “Intermetallic phases in binary Fe–W system,” Z. Metallkunde, 76, No. 1, 34–36 (1985).
F. A. Shunk, Constitution of Binary Alloys, 2nd Suppl., McGraw-Hill, New York (1969).
M. Hansen and K. Anderko, Constitution of Binary Alloys, 2nd ed., McGraw-Hill, New York (1958).
V. V. Skorokhod, V. P. Titov, and M. M. Churakov, “Interaction of tungsten with iron–copper and iron–tin melts,” Powder Metall. Met. Ceram., 48, Nos. 1–2, 1–7 (2009).
E. Lysova and L. Rokhlin, “Cu–Mo (copper–molybdenum),” in: G. Effenberg (ed.), MSIT Binary Evaluation Program, Assessment 31: Phase Diagram, Crystal Structure, Thermodynamics, Materials Science International Services GmbH, Stuttgart (2007).
V. V. Skorokhod, V. P. Titov, and M. M. Churakov, “Growth of a layer of W6Co7 phase in systems with participation of phases containing cobalt,” Powder Metall. Met. Ceram., 44, Nos. 11–12, 523–526 (2005).
V. N. Eremenko, G. M. Lukashenko, and V. L. Pritula, “Thermodynamic properties of Fe–Sn melts,” Izv. Akad. Nauk SSSR. Met., No. 1, 99–102 (1972).
M. A. Turchanin, “Formation enthalpies for liquid alloys of copper with iron, cobalt, and nickel,” Metally, No. 5, 12–19 (1995).
G. I. Batalin and V. S. Sudavtsova, “Thermodynamic properties of liquid Fe–Cu alloys,” Metally, No. 2, 43–49 (1980).
V. V. Skorokhod, V. P. Titov, Z. V. Sichkar, and M. M. Churakov, “Reaction of tungsten with melts in the Cu–Co system,” Power Metall. Met. Ceram., 44, Nos. 5–6, 271–275 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
V. V. Skorokhod is deceased.
Translated from Poroshkovaya Metallurgiya, Vol. 56, Nos. 7–8 (516), pp. 29–38, 2017.
Rights and permissions
About this article
Cite this article
Skorokhod, V.V., Titov, V.P. & Filippov, M.I. Kinetics of Reaction Interaction Between Molybdenum and Iron–Tin and Iron–Copper Melts. Powder Metall Met Ceram 56, 385–392 (2017). https://doi.org/10.1007/s11106-017-9907-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9907-3