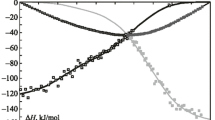
The mixing enthalpies of liquid Ce–Ni binary alloys (0 < xNi < 0.45 at 1430 K and 0.78 < xNi < 1 at 1820 K) are determined by isoperibol calorimetry. The thermodynamic properties of the liquid Ce–Ni binary alloys are calculated for the entire composition range using the model of ideal associated solutions. The thermodynamic activities of components show negative deviations from the ideal behavior. The mixing enthalpies are characterized by significant exothermic effects. The minimum mixing enthalpy of the melts is –34.8 ± 0.9 kJ/mol at xNi = 0.66 and 1820 K.







Similar content being viewed by others
References
M. I. Ivanov, V. V. Berezutskii, M. O. Shevchenko, et al., “Interaction in europium-containing alloy systems,” Dop. NAN Ukrainy, No. 8, 90–99 (2013).
W. Xiong, Y. Du, X. Lu, et al., “Reassessment of the Ce–Ni binary system supported by key experiments and ab initio calculations,” Intermetallics, 15, 1401–1408 (2007).
H. Okamoto, “Ce–Ni (cerium–nickel),” J. Phase Equilib. Diff., 30, No 4, 407 (2009).
V. S. Sudavtsov, Yu. G. Gorobets, and G. I. Batalin, “Enthalpies of forming liquid binary alloys in the Ce–(Si, Ni, Cu) systems,” Rasplavy, 2, No. 6, 79–81 (1988).
I. V. Nikolaenko and O. V. Vlasova, “Enthalpies of mixing nickel with cerium and cerium valence in melt,” Rasplavy, No. 4, 79–83 (1992).
M. Palumbo, G. Borzone, S. Delsante, et al., “Thermodynamic analysis and assessment of the Ce–Ni system,” Intermetallics, 12, 1367–1372 (2004).
Z. Du, L. Yang, and G. Ling, “Thermodynamic assessment of the Ce–Ni system,” J. Alloys Compd., 375, 186–190 (2004).
K. Yamaguchi, D.-Y. Kim, M. Ohtsuka, and K. Itagaki, “Heat content and heat of formation measurements of RNi5±x alloys (R = La, Ce, Pr or Nd) and heat balance in a reduction–diffusion process,” J. Alloys Compd., 221, 161–168 (1995).
C. Colinet and A. Pasturel, “A thermodynamic study of cerium behavior in hexagonal CeNi5 compound,” Phys. Stat. Sol. A, 80, K75 (1983).
F. Meyer-Liautaud, A. Pasturel, C. H. Allibert, and C. Colinet, “Thermodynamic study of the valence state of cerium and hydrogen storage in Ce(Ni1–x Cu x )5 compounds,” J. Less-Common Met., 110, 119–126 (1985).
B. P. Reddy, R. Babu, K. Nagarajan, and P. R. V. Rao, “Enthalpies of formation of CeNi2 and CeNi5 by calorimetry,” J. Nuclear Mater., 247, 235–239 (1997).
Q. Guo and O. J. Kleppa, “Standard enthalpies of formation of CeNi5 and of RENi (RE = Ce, Pr, Nd, Sm, Gd, Tb, Ho, Tm and Lu), determined by high-temperature direct synthesis calorimetry,” J. Alloys Compd., 270, 212–217 (1998).
Q. Guo and O. J. Kleppa, “The standard enthalpies of formation of the compounds of early transition metals with late transition metals and with noble metals as determined by Kleppa and co-workers at the University of Chicago: A review,” J. Alloys Compd., 321, 169–182 (2001).
J. Wang, J. L. Jorda, A. Pisch, and R. Flükiger, “Experimental study of the CeNi5–CeCu5 system,” Intermetallics, 14, 695–701 (2006).
A. Pisch, J. Wang, and J.-L. Jorda, “Thermodynamic modelling of the Ce–Ni system,” Int. J. Mater. Res., 97, No 6, 737–743 (2006).
M. Ivanov, V. Berezutski, and N. Usenko, “Mixing enthalpies in liquid alloys of manganese with the lanthanides,” J. Mater. Res., 102, 277 (2011).
David R. Lide, Handbook of Chemistry and Physics, 84th ed., CRC Press Taylor and Francis, Boca Raton, USA (2003).
A. T. Dinsdale, “SGTE data for pure elements,” Calphad, 15, No 4, 319–427 (1991).
D.-Y. Kim, M. Ohtsuka, and K. Itagaki, “Study on reactive diffusion in Ni–RE (RE = Ce, Pr, Nd) binary alloys,” Shigen-to-Sozai, 110, No. 2, 95–101 (1994).
R.Vogel, “On the cerium–nickel, lanthanum–nickel, praseodymium–nickel, and cerium–cobalt systems,” Z. Metallkd., 38, 97–103 (1947).
J. M. Gebhart, D. E. Etter, and P. A. Tucker, Proc. 6th R.E. Research Conference, Rare Earth Information Centre, Ames, Iowa (1967), pp. 452–457.
U. K. Duisemaliev, “Solubility in nickel and mechanical properties of nickel–cerium alloys,” Zh. Neorg. Khim., 9, 755–756 (1964).
R. H. Perkins, L. A. Geoffrian, and J. C. Biery, “Density of some low melting alloys,” Trans. AIME, 233, 1703–1710 (1965).
B. G. Qi, Z. Li, K. Itagaki, and A. Yazawa, “High temperature phase relations in Ni–RE (RE = La, Ce, Pr, Nd) binary and ternary alloy systems,” Mater. Trans. JIM, 30, No. 8, 583–593 (1989).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 54, Nos. 9–10 (505), pp. 106–115, 2015.
Rights and permissions
About this article
Cite this article
Ivanov, M.I., Berezutskii, V.V., Shevchenko, M.O. et al. Thermodynamic Properties of Ce–Ni Binary Alloys. Powder Metall Met Ceram 54, 590–598 (2016). https://doi.org/10.1007/s11106-016-9752-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-016-9752-9