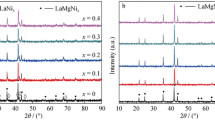
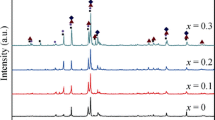
Multiphase La 2 MgNi 9 alloys are synthesized by induction melting. The crystalline structures of all phases in the alloys are determined with X-ray diffraction. The La 2 MgNi 9 electrodes with nickel as a binder and electrical conductor are prepared and their charge–discharge characteristics are studied. The influence of homogenizing annealing and mechanical grinding on the discharge capacity and cyclic stability of the metal hydride (MH) electrodes is shown. The best electrochemical parameters are observed for powdered as-cast alloys.





Similar content being viewed by others
References
J. Kleperis, G. Wójcik, A. Czerwinski, et al., “Electrochemical behavior of metal hydrides,” J. Solid State Electrochem., 5, 229–249 (2001).
O. A. Petrii and É. E. Levin, “Hydrogen storage materials in electrochemical systems,” Ros. Khim. Zh., No. 6, 115–119 (2006).
P. H. L. Notten and M. Latroche, “Secondary batteries—nickel systems, nickel–metal hydride: metal hydrides,” in: Encyclopedia of Electrochemical Power Sources, Elsevier, Amsterdam (2009), pp. 502–521.
B. Liao, Y. Q. Lei, L. X. Chen, et al., “Effect of the La/Mg ratio on the structure and electrochemical properties of La x Mg3–x Ni9 (x = 1.6–2.2) hydrogen storage electrode alloys for nickel–metal hydride batteries,” J. Power Sources, 129, 358–367 (2004).
Y. Liu, Y. Cao, L. Huang, et al., “Rare earth–Mg–Ni-based hydrogen storage alloys as negative electrode materials for Ni–MH batteries,” J. Alloys Compd., 509, No. 3, 675–686 (2011).
J. Gao, X. L. Yan, Z. Y. Zhao, et al., “Effect of annealed treatment on microstructure and cyclic stability for La–Mg–Ni hydrogen storage alloys,” J. Power Sources, 209, 257–261 (2012).
J. Zhang, B. Villeroy, B. Knosp, et al., “Structural and chemical analyses of the new ternary La5MgNi24 phase synthesized by spark plasma sintering and used as negative electrode material for Ni–MH batteries,” Int. J. Hydrogen Energy, 37, 5225–5233 (2012).
W.-K. Hu, R. V. Denys, C. C. Nwakwuo, et al., “Annealing effect on phase composition and electrochemical properties of the Co-free La2MgNi9 anode for Ni–metal hydride batteries,” Electrochim. Acta., 96, 27–33 (2013).
L. Zhang, S. Han, Y. Li, et al., “Formation mechanism, phase structure and electrochemical properties of the La–Mg–Ni-based multiphase alloys by powder sintering LaNi5 and LaMgNi4,” Int. J. Hydrogen Energy, 38, 10431–10437 (2013).
M. Latroche, F. Cuevas, W.-K. Hu, et al., “Mechanistic and kinetic study of the electrochemical charge and discharge of La2MgNi9 by in situ powder neutron diffraction,” J. Phys. Chem. C, 118, 12162–12169 (2014).
D. Zhang, J. Tang, and K. A. Gschneidner Jr., “A redetermination of the La–Ni phase diagram from LaNi to LaNi5 (50–83.3 at.% Ni),” J. Less-Common Met., 169, 45–53 (1991).
H. Inui, T. Yamamoto, Z. Di, and M. Yamaguchi, “Microstructures and defect structures in intermetallic compounds in the La–Ni alloys system,” J. Alloys Compd., 293–295, 140–145 (1999).
Z. Di, T. Yamamoto, H. Inui, and M. Yamaguchi, “Characterization of stacking faults on basal planes in intermetallic compounds La5Ni19 and La2Ni7,” Intermetallics, 8, 391–397 (2000).
F. Cuevas, M. Latroche, M. Hirscher, and A. Percheron-Guégana, “Formation and structure of highly over-stoichiometric LaNi5+x (x ~ 1) alloys obtained by manifold non-equilibrium methods,” J. Alloys Compd., 323–324, 4–7 (2001).
S. De Negri, M. Giovannini, and A. Saccone, “Phase relationships of the La–Ni–Mg system at 500°C from 66.7 to 100 at.% Ni,” J. Alloys Compd., 439, 109–113 (2007).
A. Férey, F. Cuevas, M. Latroche, et al., “Elaboration and characterization of magnesium–substituted La5Ni19 hydride forming alloys as active materials for negative electrode in Ni–MH battery,” Electrochim. Acta, 54, 1710–1714 (2009).
T. Ozaki, M. Kanemoto, T. Kakeya, et al., “Stacking structures and electrode performances of rare earth–Mg–Ni-based alloys for advanced nickel–metal hydride battery,” J. Alloys Compd., 446–447, 620–624 (2007).
Y. F. Liu, H. G. Pan, M. X. Gao, et al., “Degradation mechanism of the La–Mg–Ni-based metal hydride electrode La0.7Mg0.3Ni3.4Mn0.1,” J. Electrochem. Soc., 152, A1089–A1095 (2005).
Z. M. Wang, H. Y. Zhou, Z. F. Gu, et al., “Preparation of LaMgNi4 alloy and its electrode properties,” J. Alloys Compd., 377, L7–L9 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 54, Nos. 3–4 (502), pp. 117–125, 2015.
Rights and permissions
About this article
Cite this article
Verbovitskii, Y.V., Denis, R.V., Shtender, V.V. et al. Phase-Structural and Electrochemical Properties of La2MgNi9 Alloys. Powder Metall Met Ceram 54, 220–226 (2015). https://doi.org/10.1007/s11106-015-9703-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9703-x