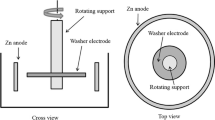
The growth of dendritic deposit under galvanostatic electrolysis is modeled and a method is developed to analyze its formation in laboratory. The time dependences for cathodic overpotential are plotted and the rod electrode with deposit is video-recorded to establish that copper dendrites form at smaller tip-radius density from the solution with surface-active agent F2 than from the solution without additions. This results in higher fluidity of the powder. Nevertheless, dendrites formed from the solution with F2 addition have greater tip radii than the deposit from the pure solution. In addition, the dendritic deposit intensively grows for a longer time with F2 addition, thus increasing the yield of powder and decreasing the yield of cathodic scrap. The results from the laboratory experiment have been confirmed by commercial production.
Similar content being viewed by others
References
O. S. Nichiporenko, Copper Powders and Alloys [in Russian], Metallurgiya, Moscow (1988), p. 206.
I. B. Murashova and A. V. Pomosov, “Electric deposition of metals as dendrites,” in: Advances in Science. Electrochemistry [in Russian], VINITI, Moscow (1989), Vol. 30, pp. 55–145.
I. B. Murashova and A. V. Pomosov, “Electric deposition of fine copper in the presence of organic additions,” Izv. Vuzov. Khim. Khim. Tekhnol., 15, No. 5, 743–745 (1972).
E. E. Usol’tseva, A. V. Pomosov, T. Yu. Kuz’mina, etc., “Electrolyte for producing copper powder by electrolysis,” USSR Inventor’s Certificate No. 1418349, Bulletin No. 31, Publ. August 23 (1988).
E. M. Natanson and Z. R. Ulberg, Colloidal Metals and Polymers [Russian translation], Naukova Dumka, Kiev (1971), p. 348.
A. P. Shpoltakov, A. T. Krest’yaninov, V. I. Chuprakov, et al., “Electrolyte for producing electrolytic copper powder,” Russian Federation Patent 2254209, Application 2004113925/02, May 5, 2004, Bulletin No. 17, Publ. June 20 (2005).
T. N. Ostanina, I. B. Murashova, and E. E. Kuz’mina, “Dynamics of growth of dendritic deposits of lead on a cylindrical electrode,” Élektrokhimiya, 32, No. 11, 1329–1333 (1996).
I. B. Murashova, P. V. Taushkanov, and N. G. Burkhanova, “Change in structural characteristics of loose copper deposit under galvanostatic electrolysis,” Élektrokhimiya, 35, No. 7, 835–840 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 49, No. 1–2 (471), pp. 3–10, 2010.
Rights and permissions
About this article
Cite this article
Murashova, I.B., Agarova, N.E., Darintseva, A.B. et al. Formation of copper deposits under electrolysis with surface-active agents. Powder Metall Met Ceram 49, 1–7 (2010). https://doi.org/10.1007/s11106-010-9194-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-010-9194-8