Abstract
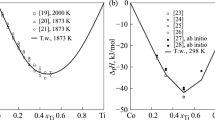
The CALPHAD method is used for the thermodynamic assessment of the Cu-Ti system that bounds the ternary Cu-Ti-Zr system, which is capable of forming amorphous alloys. The self-consistent parameters of thermodynamic models of the phases are obtained from data on the phase equilibria and thermodynamic properties of liquid alloys and intermetallic compounds. The Gibbs energy of the liquid phase is described using the associated ideal solution model. To describe the thermodynamic properties of the Cu4Ti and CuTi intermetallic compounds with homogeneity range, sublattice models are used. The calculated phase diagram of the system and the thermodynamic properties of the phases are in good agreement with experimental data.
Similar content being viewed by others
References
A. Inoue, “Stabilization of metallic supercooled liquid and bulk amorphous alloys,” Acta Mater., 48, 279–306 (2000).
Y. Zhang, D. Q. Zhao, M. X. Pan, and W. H. Wang, “Glass forming properties of Zr-based bulk metallic alloys,” J. Non-Crystalline Solids, 315, 206–210 (2003).
E. Ence and H. Margolin, “A study of the Ti-Cu-Zr system and the structure of Ti2Cu,” Trans. Metal. Soc. AIME, 221, 320–322 (1961).
C. G. Woychik and T. B. Massalski, “Phase diagram relationships in the system copper-titanium-zirconium,” Z. Metallkd., 79, No. 3, 149–153 (1988).
V. N. Chebotnikov and V. V. Molokanov, “Structure and properties of alloys of the Ti2Cu-Zr2Cu section of the Ti-Zr-Cu system in amorphous and crystalline states,” Izv. AN SSSR, Neorg. Mater., 26, No. 5, 960–964 (1990).
Yu. K. Kovneristyi and A. G. Pashkovskaya, “Bulk amorphization of alloys of the Ti-Cu-Zr system which includes intermetallic compounds,” in: Amorphization (Glass Formation) of Metallic Materials [in Russian], Inst. Metallurgii RAN, Moscow (1992), pp. 153–157.
A. M. Storchak-Fedyuk, V. M. Petyukh, and L. V. Artyukh, “Study of cast alloys of the Cu-Ti-Zr system,” in: Modern Problems of Materials Science, Ser. Physical and Chemical Fundamentals of Powder Technology [in Russian], Kiev (2007), pp. 22–26.
A. Inoue, W. Zhang, T. Zhang, and K. Kurosaka, “High-strength Cu-based bulk glassy alloys in Cu-Zr-Ti and Cu-Hf-Ti ternary systems,” Acta Mater., 49, No. 14, 2645–2652 (2001).
T. Shindo, Y. Waseda, and A. Inoue, “Prediction of critical compositions for bulk glass formation in Labased, Cu-based and Zr-based ternary alloys,” Mater. Trans., JIM, 44, No. 3, 351–357 (2003).
Q. S. Zhang, H. F. Zhang, Y. F. Deng, et al., “Bulk metallic glass formation of Cu-Zr-Ti-Sn alloys,” Scr. Mater., 49, No. 4, 273–278 (2003).
R. Arroyave, T. W. Eagar, and L. Kaufman, “Thermodynamic assessment of the Cu-Ti-Zr system,” J. Alloys Compd., 351, No. 1–2, 158–170 (2003).
T. Velikanova and M. A. Turchanin, “Cu-Ti-Zr,” in: Ternary Alloy Systems. Phase Diagrams, Crystallographic and Thermodynamic Data, G. Effenberg and S. Ilyenko (eds.), Vol. 11C3, Springer-Verlag, Germany, Berlin, Heidelberg (2006), pp. 436–464.
A. R. Abdulov, M. A. Turchanin, P. G. Agraval, and A. A. Solorev, “Mixing enthalpy of liquid alloys of the Cu-Ti-Zr system,” Metally, No. 1, 28–34 (2007).
U. Thiedermann, M. Rosner-Kuhn, K. Drewes, et al., “Mixing enthalpy measurements of liquid Ti-Zr, Fe-Ti-Zr, and Fe-Ni-Zr alloys,” J. Steel Research, 70, No. 1, 3–8 (1999).
M. A. Turchanin, P. G. Agraval, A. N. Fesenko, and A. R. Abdulov, “Thermodynamics of liquid alloys and metastable phase transformations in the copper-titanium system,” Powder Metall. Met. Ceram., 44, No. 5–6, 259–270 (2005).
A. A. Turchanin, I. A. Tomilin, M. A. Turchanin, et al., “Enthalpies of formation of liquid and amorphous Zr-Cu alloys,” J. Non-Crystalline Solids, 250–252, 582–585 (1999).
A. D. McQuillan, “The application of hydrogen equilibrium-pressure measurements to the investigation of titanium alloy systems,” J. Ins. Metals., 79, 73–88 (1951).
A. Joukainen, N. J. Grant, and C. F. Floe, “Titanium-copper binary phase diagram,” J. Metals, 4, 766–770 (1952).
E. Raub, P. Walter, and H. Engel, “Alloys of titanium with copper, silver and gold,” Z. Metallkd., 43, 112–118 (1952).
W. Trzebiatowski, J. Berak, and T. Romotowski, “The copper-titanium system,” Roczniki Chemii, 27, 426–437 (1953).
V. N. Vigdorovich, A. N. Krestovnikov, and M. V. Mal’tsev, “Microhardness investigation of solid solutions of ternary systems,” Izvest. Akad. Nauk SSSR. Otdel. Tekh. Nauk, No. 3, 110–113 (1958).
M. J. Saarivirta and H. S. Cannon, “Copper-titanium alloys,” Metal Progress, 76, No. 2, 81–84 (1959).
K. P. Kalinin and M. Z. Spiridonov, “Studying the properties of copper-titanium alloys,” Tr. Gos. Nauchn.-Issled. Proektn. Inst. Obrab. Tsvetn. Metal., 18, 46–57 (1960).
P. Pietrokowsky and J. R. Maticich, “Use of the electron microprobe analyzer in the study of binary metal alloy systems,” in: Proc. 3rd Int. Symp. X-Ray Opt. X-Ray Microanalysis, Stanford (1963), pp. 591–602.
K. Schubert, “About titanium-copper and titanium-silver systems,” Z. Metallkd., 56, No. 3, 197–199 (1965).
V. N. Eremenko, Yu. I. Buyanov, and S. B. Prima, “Phase diagram of the system titanium-copper,” Powder Metall. Met. Ceram., 5, No. 6, 494–502 (1966).
V. N. Eremenko, Yu. I. Buyanov, and N. M. Panchenko, “Polythermal and isothermal sections of the system titanium-copper-silver,” Powder Metall. Met. Ceram., 9, No. 5, 410–414 (1970).
R. C. Ecob, J. V. Bee, and B. Ralph, “The structure of the β-phase in dilute copper-titanium alloys,” Physica Stat. Sol. A, 52, No. 1, 201–210 (1979).
G. Vigier, J. M. Pelletier, and J. Merlin, “Determination of copper solubility in titanium and study of titanium-copper solid solution stability by thermoelectric power measurements,” J. Less-Common Met., 64, No. 2, 175–183 (1979).
K. Nagata and S. Nishikawa, “Aging and reversion phenomena of copper-titanium alloy,” in: Report of the Institute of Industrial Science, University of Tokyo, 29, No. 4, p. 40 (1981).
J.-Y. Brun, S.-J-H. Thibault, and C.-H. Allibert, “Cu-Ti and Cu-Ti-Al solid state phase equilibria in the copper-rich region,” Z. Metallkd., 74, No. 8, 525–529 (1983).
V. N. Eremenko, R. N. Mogilevskii, V. M. Sergeenkova, and V. M. Petyuukh, “Melting heat and homogeneity range of TiCu intermetallic compound,” Izv. AN SSSR, Metally, No. 6, 171–173 (1988).
S. P. Alisova, N. V. Lutskaya, A. N. Kobylkin, and P. B. Budberg, “The TiFe-Ti2Cu section of the Ti-Fe-Cu system. Conditions for the formation of Ti2Cu,” Metally, No. 5, 170–172 (1994).
T. Yamane, S. Nakajima, H. Araki, et al., “Partial phase diagrams of the titanium-rich region of the Ti-Cu system under high pressure,” J. Mater. Sci. Lett., 13, No. 3, 162–164 (1994).
V. E. Oliker, A. A. Mamonova, and T. I. Shaposhnikova, “Structure and phase composition of the Ti-Cu diffusion zone,” Powder Metall. Met. Ceram., 35, No. 3–4, 173–175 (1996).
S. Nagarjuna and D. S. Sarma, “On the variation of lattice parameter of Cu solid solution with solute content in Cu-Ti alloys,” Scr. Mater., 41, No. 4, 359–363 (1999).
P. Canale and C. Servant, “Thermodynamic assessment of the Cu-Ti system taking into account the new stable phase CuTi3,” Z. Metallkd., 93, 273–276 (2002).
J. L. Murray, “Cu-Ti (copper-titanium),” in: Phase Diagrams of Binary Copper Alloys, ASM, Ohio (1994), pp. 447–460.
N. Karlsson, “An x-ray study of the phases in the copper-titanium system,” J. Inst. Met., 79, 391–405 (1951).
M. H. Mueller and H. W. Knott, “The Crystal Structures of Ti2Cu, Ti2Ni, Ti4Ni2O, and Ti4Cu2O,” Trans. Metall. Soc. AIME, 227, 674–678 (1963).
G. Lutjerung and S. Weissmann, “Mechanical properties and structure of age-hardened Ti-Cu alloys,” Metal. Trans., 1, No. 6, 1641–1649 (1970).
M. A. Turchanin, I. V. Belokonenko, and P. G. Agraval, “Applying the ideal associated-solution theory to the description of the temperature-concentration dependence of the thermodynamic properties of binary melts,” Rasplavy, No. 1, 58–69 (2001).
M. Arita, R. Kinaka, and M. Someno, “Application of the metal-hydrogen equilibration for determining thermodynamic properties in the titanium-copper system,” Met. Trans. A, 10, No. 5, 529–534 (1979).
O. I. Kleppa and S. Watanabe, “Thermochemistry of alloys of transition metals. Part III. Copper-silver,-titanium,-zirconium and-hafnium at 1373 K,” Met. Trans. B, 13, No. 1, 391–401 (1982).
C. Colinet, A. Pasturel, and K. H. J. Buschow, “Enthalpies of formation of Ti-Cu intermetallic and amorphous phases,” J. All. Comp., 247, 15–19 (1997).
L. Kaufman, “Coupled phase diagrams and thermochemical data for transition metal binary systems-III,” CALPHAD, 2, No. 2, 117–146 (1978).
H. Kumar, I. Ansara, P. Wollants, and L. Delaey, “Thermodynamic optimization of the Cu-Ti system,” Z. Metallkd., 87, No. 8, 666–672 (1996).
M. A. Turchanin and S. V. Porokhnya, “Enthalpy of formation of liquid alloys of copper with titanium and zirconium,” Rasplavy, No. 5, 29–32 (1995).
A. T. Dinsdale, “SGTE data for pure elements,” CALPHAD, 15, No. 4, 317–425 (1991).
J.-O. Andersson, A. F. Guillermet, M. Hillert, et al., “A compound-energy model of ordering in a phase with sites of different coordination numbers,” Acta Metall., 34, 437–445 (1986).
N. Saunders and A. P. Miodownik, CALPHAD (Calculation of Phase Diagrams), in: Comprehensive Guide, Vol. 1, Pergamon Materials Series (1998), p. 496.
M. Hillert, “The compound energy formalism,” J. Alloys Compd., 320, 161–176 (2001).
I. Ansara, T. G. Chart, A. Fernandez Guillermet, et. al., “Group 2: Alloy system I thermodynamic modeling of selected topologically close-packed intermetallic compounds,” CALPHAD, 21, No. 2, 171–218 (1997).
T. B. Massalski, H. Okamoto, P. R. Subramanian, and L. Kacprzak (eds.), Binary Alloys Phase Diagrams, 2nd ed., ASM International, Ohio (1990), p. 3589.
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Poroshkovaya Metallurgiya, Vol. 47, No. 3–4 (460), pp. 102–121, 2008.
Rights and permissions
About this article
Cite this article
Turchanin, M.A., Agraval, P.G. & Abdulov, A.R. Thermodynamic assessment of the Cu-Ti-Zr system. I. Cu-Ti system. Powder Metall Met Ceram 47, 344–360 (2008). https://doi.org/10.1007/s11106-008-9026-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-008-9026-2