Abstract
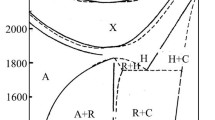
We have used x-ray phase analysis, electron-probe microanalysis, petrography, and electron microscopy on annealed specimens to study phase equilibria in the ternary system HfO2-Y2O3-La2O3 at 1900 °C over the entire concentration range. We have plotted the isothermal cross section of the phase diagram for this system at the indicated temperature. We found 23 phase regions. A typical feature of the system is formation of solid solutions based on different crystal modifications of the starting components (A-and H-La2O3, C-Y2O3, T-and F-HfO2) and also the compounds La2Hf2O7, LaYO3. We did not observe new phases in the system. The nature of the phase equilibria in the system is consistent with the high relative thermodynamic stability of lanthanum hafnate (ΔH °La2Hf2O7 ≈ 100 kJ/mole) compared with LaYO3. We established that adding a third component extends the thermal stability region for the ordered phase of LaYO3 toward higher temperatures.
Similar content being viewed by others
References
J. Wang, R. Stevens, and H. P. Li, “Review of hafnia and hafnia-toughened ceramics,” J. Mat. Sci., 27, 5397–5430 (1992).
H. Shiromizu and N. Morita, “Properties, areas of application, and prospects for development of technical ceramic,” Seidenki Gakkaishi, Proc. Inst. Electrost. Jpn., 10, No. 6, 426–429 (1986).
I. Hiroyuki, I. Naboru, T. Hiroshi, et al., Material for a Seal between Ceramic Pieces, Ceramic and Metal, Pat. 58-41766 Japanese, Publ. 1981.
E. R. Andrievskaya, L. M. Lopato, V. V. Kovylyaev, and Z. A. Zaitseva, “Phase equilibria in the ternary system HfO2-Y2O3-La2O3,” Neorg. Mater., 32, No. 6, 727–735 (1996).
E. R. Andrievskaya, L. M. Lopato, V. P. Smirnov, and I. E. Kir’yakova, “Isothermal cross section of the phase diagram for the system HfO2-Y2O3-La2O3 at 1600°C,” Poroshk. Metall., Nos. 7–8, 147–158 (1996).
E. R. Andrievskaya, L. M. Lopato, and I. E. Kir’yakova, “Solid-phase transformations in the systems HfO2-Y2O3-Ln2O3 (Ln = La, Er): model and experiment,” in: Physical Materials Science and the Physicochemical Principles for Design of New Materials [in Russian], V. V. Skorokhod (ed.), Institute for Problems of Materials Science, National Academy of Sciences of Ukraine, Kiev (1994), pp. 85–95.
E. R. Andrievskaya, L. M. Lopato, and A. V. Shevchenko, “Phase transformations in the ternary systems HfO2(ZrO2)-Y2O3-La2O3(Er2O3),” in: Proceedings, Fourth European Ceramic Society Conference on Basic Science in Processing of Advanced Ceramics (October 2–6, 1995, Italy), Faenza (1995), Vol. 2, pp. 431–439.
J. Coutures and M. Foex, “High temperature study of the phase diagram of the system formed by yttrium sesquioxide,” J. Solid State Chem., 11, No. 4, 294–300 (1974).
J. Coutures, F. Sibieude, and M. Foex, “High temperature study of systems formed by lanthanum sesquioxides with lanthanide sesquioxides. II. Effect of quenching on the nature of the phases obtained at room temperature,” J. Solid State Chem., 17, No. 4, 377–384 (1976).
M. Mizuno, A. Rouanet, T. Yamada, and T. Noguchi, “Phase diagram of the system La2O3-Y2O3 at high temperature,” J. Ceram. Soc. Japan, 84, No. 7, 342–347 (1976).
L. M. Lopato, B. S. Nigmanov, A. V. Shevchenko, and Z. A. Zaitseva, “Reaction of lanthanum oxide with yttrium oxides,” Izv. Akad. Nauk SSSR, Neorg. Mater., 22, No. 5, 771–774 (1986).
G. C. Wei, T. Emma, and W. H. Rhodes, “Analytical microscopy study of phases and fracture in Y2O3-La2O3 alloys,” J. Amer. Ceram. Soc., 71, No. 10, 820–825 (1988).
J. Coutures, A. Rouanet, R. Verges, and M. Foex, “High temperature study of systems formed by lanthanum sesquioxide and lanthanide sesquioxides. I. Phase diagrams (1400°C < T < T liquid),” J. Solid State Chem., 17, No. 1–2, 172–182 (1976).
V. Berndt, D. Maier, and C. Keller, “New A‴B‴O3 interlanthanide perovskite compounds,” J. Solid State Chem., 13, No. 1–2, 131–135 (1975).
W. H. Rhodes, “Controlled transient solid second phase sintering of yttria,” J. Amer. Ceram. Soc., 64, No. 1, 13 (1981).
P. Duran, “Phase relationships in the systems HfO2-La2O3 and HfO2-Nd2O3,” Ceram. Intern., 1, No. 1, 10–13 (1975).
A. V. Shevchenko and L. M. Lopato, “Effect of hafnium dioxide on the polymorphism of lanthanoid oxides,” Dop. AN UkrSSR, Ser. B, No. 8, 737–739 (1975).
É. L. Karyakina, E. I. Zoz, A. M. Gavrish, and N. V. Gul’ko, “Some crystal chemistry and thermophysical characteristics of lanthanum zirconate and hafnate,” Zhurn. Neorg. Khim., 23, No. 12, 3202–3205 (1978).
A. V. Shevchenko and L. M. Lopato, “Effect of lanthanoid oxides of the cerium subgroup on the polymorphism of hafnium dioxide,” Dop. AN UkrSSR, Ser. B, No. 8, 718–721 (1977).
A. V. Shevchenko, L. M. Lopato, A. I. Stegnii, et al., “Liquidus of hafnium dioxide — rare earth oxide systems in the high HfO2 content region,” Izv. Akad. Nauk SSSR, Neorg. Mater., 17, No. 6, 1022–1020 (1981).
A. V. Shevchenko, L. M. Lopato, and Z. A. Zaitseva, “Reaction of HfO2 with lanthanum, praseodymium, and neodymium oxides at high temperatures,” Izv. Akad. Nauk SSSR, Neorg. Mater., 20, No. 9, 1530–1534 (1984).
V. B. Glushkova, M. V. Kravchinskaya, A. K. Kuznetsov, and P. A. Tikhonov, Hafnium Dioxide and Its Compounds with Rare Earth Oxides [in Russian], Nauka, Leningrad (1984).
A. V. Shevchenko, L. M. Lopato, and I. E. Kir’yakova, “Reaction of HfO2 with Y2O3, Ho2O3, Er2O3, Tm2O3, Yb2O2, and Lu2O3 at high temperatures,” Izv. Akad. Nauk SSSR, Neorg. Mater., 20, No. 12, 1991–1996 (1984).
M. F. Trubelja and V. S. Stubican, “Phase equilibria and ordering in the system Zirconia-Hafnia-Yttria,” J. Amer. Ceram. Soc., 71, No. 8, 662–666 (1988).
P. A. Arsen’ev, V. B. Glushkova, et al., Compounds of Rare Earth Elements. Zirconates, Hafnates, Niobates, Tantalates, Antimonates [in Russian], Nauka, Moscow (1985).
V. K. Pecharskii, P. Yu. Zavalii, L. G. Ansel’rud, et al., “Structural analysis software package for the UVKSM-4,” Vest. L’vov. Univ., Ser. Khim., No. 25, 9–11 (1984).
L. H. Ahrens, “Use of ionization potentials. I. Ionic radii of the elements,” Geochem. Cosmochim. Acta, No. 2, 155–158 (1952).
Author information
Authors and Affiliations
Additional information
__________
Translated from Poroshkovaya Metallurgiya, Nos. 1–2(447), pp. 73–87, January–February, 2006.
Rights and permissions
About this article
Cite this article
Andrievskaya, E.R., Lopato, L.M. & Smirnov, V.P. Phase equilibria in the system HfO2-Y2O3-La2O3 AT 1900°C. Powder Metall Met Ceram 45, 59–71 (2006). https://doi.org/10.1007/s11106-006-0042-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11106-006-0042-9