Abstract
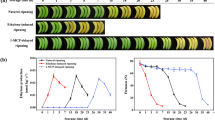
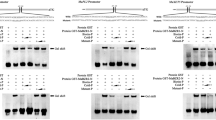
The LATERAL ORGAN BOUNDARIES (LOB) DOMAIN (LBD) proteins are plant-specific transcriptional factors (TFs) functioning in the growth and development of Arabidopsis and other plant species. However, the involvement of LBD TFs in regulating the ripening of economically important fruits is largely unknown. In the present study, four full-length LBD genes, designated as MaLBD1–MaLBD4, were isolated and characterized from banana fruit. Expressions of MaLBD1–MaLBD4 in fruit with three ripening characteristics revealed that MaLBD1/2/3 were ethylene-inducible, and their transcript levels obviously increased during fruit ripening, while MaLBD4 changed slightly. Moreover, the activities of MaLBD1/2/3 promoters were activated after ethylene treatment, further supporting their involvement in fruit ripening. Subcellular localization showed that MaLBD1/2/3 were nuclear proteins, and a transactivation assay in protoplasts demonstrated that MaLBD1/2/3 had transactivation activity. More importantly, a transient expression assay further indicated that MaLBD1/2/3 were transcriptional activators that regulated ripening-related MaEXP1/2 expressions by directly binding to MaEXP1/2 promoters. These results suggest that MaLBDs are involved in regulating fruit ripening, in part by transcriptional activation of the EXPANSIN expression related to cell wall modification. Taken together, our findings provide some new information about the functions of LBD TFs involved in the regulation of fruit ripening.







Similar content being viewed by others
References
Abel S, Theologis A (1994) Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J 5:421–427
Bapat VA, Trivedi PK, Ghosh A, Sane VA (2010) Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnol Adv 28:94–107
Chen L, Zhong HY, Kuang JF, Li JG, Lu WJ, Chen JY (2011) Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234:377–390
Choudhury SR, Roy S, Nag A, Singh SK, Sengupta DN (2012) Characterization of an AGAMOUS-like MADS box protein, a probable constituent of flowering and fruit ripening regulatory system in banana. PLoS One 7:e44361
Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407:321–326
Cruz-Hernández A, Paredes-López O (2012) Fruit quality: new insights for biotechnology. Crit Rev Food Sci Nutr 52:272–289
D’Hont A, Denoeud F, Aury JM, Baurens FC, Carreel F, Garsmeur O et al (2012) The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488:213–217
Elitzur T, Vrebalov J, Giovannoni JJ, Goldschmidt EE, Friedman H (2010) The regulation of MADS-box gene expression during ripening of banana and their regulatory interaction with ethylene. J Exp Bot 61:1523–1535
El-Sharkawy I, Sherif S, Mila I, Bouzayen M, Jayasankar S (2009) Molecular characterization of seven genes encoding ethylene responsive transcriptional factors during plum fruit development and ripening. J Exp Bot 60:907–922
Gapper NE, McQuinn RP, Giovannoni JJ (2013) Molecular and genetic regulation of fruit ripening. Plant Mol Biol 82:575–591
Guo M, Thomas J, Collins G, Timmermans MC (2008) Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20:48–58
Hellens R, Allan A, Friel E, Bolitho K, Grafton K, Templeton M, Karunairetnam S, Gleave A, Laing W (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1:13
Husbands A, Bell EM, Shuai B, Smith HMS, Springer PS (2007) LATERAL ORGAN BOUNDARIES defines a new family of DNA-binding transcription factors and can interact with specific bHLH proteins. Nucleic Acids Res 35:6663–6671
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Kuang JF, Chen JY, Luo M, Wu KQ, Sun W, Jiang YM, Lu WJ (2012) Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J Exp Bot 63:441–454
Lee HW, Kim J (2013) EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant Cell Physiol 54:1600–1611
Lee HW, Kim MJ, Kim NY, Lee SH, Kim J (2013) LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J 73:212–224
Lee HW, Kim NY, Lee DJ, Kim J (2009) LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol 151:1377–1389
Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43:47–56
Majer C, Hochholdinger F (2011) Defining the boundaries: structure and function of LOB domain proteins. Trends Plant Sci 16:47–52
Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ (2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157:1568–1579
Matsumura Y, Iwakawa H, Machida Y, Machida C (2009) Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J 58:525–537
Mbéguié-A-Mbéguié D, Hubert O, Baurens FC, Matsumoto T, Chillet M, Fils-Lycaon B, Sidibé-Bocs S (2009) Expression patterns of cell wall-modifying genes from banana during fruit ripening and in relationship with finger drop. J Exp Bot 60:2021–2034
Naito T, Yamashino T, Kiba T, Koizumi N, Kojima M et al (2007) A link between cytokinin and ASL9 (ASYMMETRIC LEAVES 2 LIKE 9) that belongs to the AS2/LOB (LATERAL ORGAN BOUNDARIES) family genes in Arabidopsis thaliana. Biosci Biotechnol Biochem 71:1269–1278
Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19:118–130
Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14:399–408
Rast MI, Simon R (2012) Arabidopsis JAGGED LATERAL ORGANS acts with ASYMMETRIC LEAVES2 to coordinate KNOX and PIN expression in shoot and root meristems. Plant Cell 24:2917–2933
Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR (2009) Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21:3567–3584
Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S et al (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326:1112–1115
Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64:219–241
Shan W, Kuang JF, Chen L, Xie H, Peng HH, Xiao YY, Li XP, Chen WX, He QG, Chen JY, Lu WJ (2012) Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J Exp Bot 63:5171–5187
Shuai B, Reynaga-Peña CG, Springer PS (2002) The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol 129:747–761
Solano R, Stepanova A, Chao QM, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12:3703–3714
Soyano T, Thitamadee S, Machida Y, Chua NH (2008) ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis. Plant Cell 20:3359–3373
Thatcher LF, Powell JJ, Aitken EA, Kazan K, Manners JM (2012a) The lateral organ boundaries domain transcription factor LBD20 functions in Fusarium wilt susceptibility and jasmonate signaling in Arabidopsis. Plant Physiol 160:407–418
Thatcher LT, Kazan K, Manners JM (2012b) Lateral organ boundaries domain transcription factors: new roles in plant defense. Plant Signal Behav 7:1702–1704
Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223:7–12
Wang A, Tan DM, Takahashi A, Li TZ, Harada T (2007) MdERFs, two ethylene-response factors involved in apple fruit ripening. J Exp Bot 58:3743–3748
Wang XF, Zhang SZ, Su L, Liu X, Hao YJ (2013) A genome-wide analysis of the LBD (LATERAL ORGAN BOUNDARIES domain) gene family in Malus domestica with a functional characterization of MdLBD11. PLoS One 8:e57044
Xiao YY, Chen JY, Kuang JF, Shan W, Xie H, Jiang YM, Lu WJ (2013) Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot 64:2499–2510
Yang Y, Yu X, Wu P (2006) Comparison and evolution analysis of two rice subspecies LATERAL ORGAN BOUNDARIES domain gene family and their evolutionary characterization from Arabidopsis. Mol Phylogenet Evol 39:248–262
Yin XR, Allan AC, Chen KS, Ferguson IB (2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol 153:1280–1292
Yordanov YS, Regan S, Busov V (2010) Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell 22:3662–3677
Zhu QH, Guo AY, Gao G, Zhong YF, Xu M, Huang M, Luo J (2007) DPTF: a database of poplar transcription factors. Bioinformatics 23:1307–1308
Acknowledgments
We thank Professor Seiichiro Hasezawa (Department of Integrated Biosciences, the University of Tokyo), Professor Shouyi Chen (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences), and Professor Junping Gao (Department of Ornamental Horticulture, China Agricultural University) for the generous gift of tobacco BY-2 suspension cells and the transient expression vectors, respectively. This work was supported in part by the National Natural Science Foundation of China (grant no. 31372111), the China Agriculture Research System (grant no. CARS-32-09) and Guangdong Modern Agricultural Industry Technology System (grant no. LNSG2011-12).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ba, Lj., Shan, W., Kuang, Jf. et al. The Banana MaLBD (LATERAL ORGAN BOUNDARIES DOMAIN) Transcription Factors Regulate EXPANSIN Expression and Are Involved in Fruit Ripening. Plant Mol Biol Rep 32, 1103–1113 (2014). https://doi.org/10.1007/s11105-014-0720-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-014-0720-6