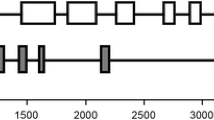
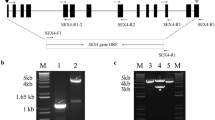
Abstract
ADP-glucose pyrophosphorylase catalyzes the first and limiting step in starch biosynthesis. Six cDNA sequences encoding three large subunits and three small subunits of maize AGPase from database were mined and subsequently named: agpl1, agpl2, agpl3, agps1, agps2, and agps3. To elucidate the roles of these isogenes, a comprehensive expression analysis of the gene family was conducted by quantitative real-time RT-PCR. Based on the expression patterns, the six genes can be divided into three groups: (1) steady expressers (agpl1, agps1, and agpl2), which were expressed relatively constantly both in leaf and grain; (2) tissue and development-specific expressers (agpl3 and agps2), which were expressed only in grain at middle and late development phases; (3) tissue-specific expressers (agps3), whose transcripts kept constant during grain filling and were observed only in grain. In order to clarify the effects of sugar and plant hormone on maize AGPase genes expression, a serial of treatments were used. The results showed that AGPase genes significantly differed in response to sugar and hormone inductions. Enormous transcript changes of these genes could be observed in glucose and sucrose treatments. Interestingly, synergistic effect of ABA and sucrose on these genes was observed.








Similar content being viewed by others
Abbreviations
- DAP:
-
Day after pollination
- AGPase:
-
ADP-glucose pyrophosphorylase
- ADPG:
-
Adenosine diphosphate glucose
- Glc-1-P:
-
Glucose-1-phosphate
- Glc-6-P:
-
Glucose-6-phosphate
- bt1 :
-
Brittle 1
- sh2 :
-
Shrunken 2
- bt2 :
-
Brittle 2
- ABA:
-
Abscisic acid
- BA:
-
N(6)-benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- Pi:
-
Orthophosphate
- PGA:
-
3-phosphoglycerate
References
Akihiro T, Mizuno K, Fujimura T (2005) Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol 46(6):937–946
Burton RA, Johnson PE, Beckles DM et al (2002) Characterization of the genes encoding the cytosolic and plastidial forms of ADP-glucose pyrophosphorylase in wheat endosperm. Plant Physiol 130:1464–1475
Burger B, Cross J, Okita TW et al (2003) Relative turnover numbers of maize endosperm and potato tuber ADPglucose pyrophosphorylases in the absence and presence of 3-PGA. Planta 217:449–456
Crevillén P, Ventriglia T, Pinto F et al (2005) Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J Biol Chem 280:8143–8149
Chen BY, Janes HW, Gianfagna T (1998) PCR cloning and characterization of multiple ADP-glucose pyrophosphorylase cDNAs from tomato. Plant Sci 136(1):59–67
Cossegal M, Chambrier P, Mbelo S et al (2008) Transcriptional and metabolic adjustments in ADP-glucose pyrophosphorylase-deficient bt2 maize kernels. Plant Physiol 146(4):1553–1570
Denyer K, Dunlap F, Thorbjornsen T et al (1996) The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiol 112(2):779–785
Dickinson DB, Preiss J (1969) Presence of ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiol 44:1058–1062
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Frueauf JB, Ballicora MA, Preiss J (2001) Aspartate residue142 is important for catalysis by ADP-glucose pyrophosphorylase from Escherichia coli. J Biol Chem 276:46319–46325
Frueauf JB, Ballicora MA, Preiss J (2003) ADP-glucose pyrophosphorylase from potato tuber: site-directed mutagenesis of homologous aspartic acid residues in the small and large subunits. Plant J 33(3):503–511
Giroux M, Hannah LC (1994) ADP glucose pyrophosphorylase in shurunken-2 and brittle-2 mutants of maize. Mol Gen Genet 243:400–408
Harn CH, Bae JM, Lee SS et al (2000) Presence of multiple cDNAs encoding an isoform of ADP-glucose pyrophosphorylase large subunit from sweet potato and characterization of expression levels. Plant Cell Physiol 41(11):1235–1242
Hwang SK, Hamada S, Okita TW (2006) ATP binding site in the plant ADP-glucose pyrophosphorylase large subunit. FEBS Lett 580:6741–6748
Hwang SK, Hamada S, Okita TW (2007) Catalytic implications of the higher plant ADP-glucose pyrophosphorylase large subunit. Phytochemistry 68(4):464–477
Kleczkowski LA, Villand P, Preiss J (1993a) Kinetic mechanism and regulation of ADP-glucose pyrophosphorylase from barley (Hordeum vulgare) leaves. J Biol Chem 268:6228–6233
Kleczkowski LA, Villand P, Luthi E et al (1993b) Insensitivity of barley endosperm ADP-glucose pyrophosphorylase to 3-phosphoglycerate and orthophosphate regulation. Plant Physiol 101(1):179–186
Kwak MS, Noh SA, Oh MJ et al (2006) Two sweet potato ADP-glucose pyrophosphorylase isoforms are regulated antagonistically in response to sucrose content in storage roots. Gene 336(1):87–96
Muller-Rober BT, Kossmann J, Hannah LC et al (1990) one of two different ADPglucose pyropho-sphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet 224:136–146
Müller-Röber B, Sonnewald U, Willmitzer L (1992) Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11(4):1229–1238
Ohdan T, Francisco PB, Sawada T et al (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56:3229–3244
Preiss J (1984) Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol 38:419–458
Preiss J, Ball K, Smith-White B et al (1991) Starch biosynthesis and its regulation. Biochem Soc Trans 19(3):539–547
Prioul JL, Jeannette E, Reyss A et al (1994) Expression of ADPglucose pyrophosphorylase in maize (Zea mays L.) grain and source leaf during grain filling. Plant Physiol 104:179–187
Plaxton WC, Preiss J (1987) Purification and properties of nonproteolytic degraded ADPglucose pyrophosphorylase from maize endosperm. Plant Physiol 83:105–112
Rösti S, Denyer K (2007) Two paralogous genes encoding small subunits of ADP-glucose pyrophosphorylase in maize, Bt2 and L2, replace the single alternatively spliced gene found in other cereal species. J Mol Evol 65(3):316–327
Sivak MN, Preiss J (1998) Starch: basic science to biotechnology. In: Taylor SL (ed) Advances in Food and Nutrition Research, pp 1–199. Academic Press, San Diego, California
Sowokinos JR (1981) Pyrophosphorylases in Solanum tuberosum II. Catalytic properties and regulation of ADPglucose and UDPglucose pyrophosphorylase activity in potato. Plant Physiol 68:924–929
Sweetlove LJ, Müller-Röber B, Willmitze L et al (1999) The contribution of adenosine 5′-diphosphoglucose pyrophosphorylase to the control of starch synthesis in potato tubers. Planta 209(3):330–337
Sullivan TD (1991) Analysis Of the maize brittle-1 alleles and a defective suppressor mutator induced mutable allele. Plant Cell 3:1337–1348
Sullivan TD (1995) The maize brittle 1 gene encodes amyloplast membrane poly peptides. Planta 196:477–484
Shannon JC, Pien FM (1996) Nucleotides and nucleotide sugars in developing maize endosperms. Plant Physiol 110:835–843
Sokolov LN, Dejardin A, Kleczkowski LA (1998) Sugars and light/dark exposure trigger differential regulation of ADP-glucose pyrophosphorylase genes in Arabidopsis thaliana (thale cress). Biochem J 336:681–687
Tetlow IJ, Davies EJ, Vardy KA et al (2003) Subcellular localization of ADPglucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform. J Exp Bot 54(383):715–725
Tobias RB, Boyer CD, Shannon JC (1992) Alterations in carbohydrate intermediates in the endosperm of starch-deficient maize (Zea mays L.) genotypes. Plant Physiol 99:146–152
Acknowledgments
This work was supported by National Natural Science Foundation of China (31071354), National High Technology Research and Development Program 863 in China (‘863 program’ Grant No: 2008AA10Z123) and the National Transgenic Major Program(2009ZX08003-022B). The authors are very grateful to Professor Alan Myers and Dr. Tracie Hennen-Bierwagen (Iowa State University) for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 470 kb)
Rights and permissions
About this article
Cite this article
Huang, B., Chen, J., Zhang, J. et al. Characterization of ADP-Glucose Pyrophosphorylase Encoding Genes in Source and Sink Organs of Maize. Plant Mol Biol Rep 29, 563–572 (2011). https://doi.org/10.1007/s11105-010-0262-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-010-0262-5