Abstract
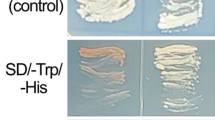
Proteomic and transcriptomic analyses of pathogenesis-related proteins, PR10, were carried out by subjecting adult plants of the salt-tolerant grapevine cv. Razegui to 100 mM NaCl for 6 and 24 h under controlled greenhouse conditions. Within 6 h of salt exposure, an increase in levels of RzPR10 was observed in the roots. PR10 mRNA levels in leaves were significantly different than those in roots; moreover, significant differences for mRNA levels were found between treated and control plants. Within 24 h of salt treatment, roots exhibited the highest levels of RzPR10 protein, but no differences were observed for mRNA levels in both leaves and roots. Overexpression of Vvpr10 cDNA, under the control of the Gal promoter, in the yeast Saccharomyces cerevisiae revealed that growth of transformed cells on a minimal medium containing 500 mM NaCl is similar to that of cells cultivated on NaCl-free medium. This indicated that Vvpr10 gene was functional in yeast and conferred tolerance to high salt concentrations.




Similar content being viewed by others
References
Balsamo RA, Wang JL, Eckard KJ, Wang CS, Lord EM (1995) Immunoglod localization of a developmentally regulated, tapetal-specific, 15 kDa lily protein. Protoplasma 17:25–189
Bantignies B, Seguin J, Muzac I, Dadaldechamp F, Guilick P, Ibrahim R (2000) Direct evidence for rbonucleolytic activity of a PR-10-like protein from white lupin roots. Plant Mol Biol 871:881–42
Bonomelli A, Mercier L, Franchel J, Baillieul F, Benizri E, Mauro MC (2004) Response of grapevine defenses to UV-C exposure. Am J Enol Vitic 51:59–55
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principale of protein-due binding. Ann Biochem 72:248–54. doi:10.1016/0003-2697(76)90527-3
Bressan RA, Hasegawa PM, Pardo JM (1998) Plants use calcium to resolve salt stress. Trends Plant Sci 3:411–2. doi:10.1016/S1360-1385(98)01331-4
Bufe A, Spangfort MD, Kahlert H, Schlaak M, Becker WM (1996) The major birch pollen allergen, Bet v1, shows ribonuclease activity. Planta 188:289–95
Castro AJ, Carapito C, Zorn N, Magne C, Leize E, Dorsselaer AV, Clément C (2005) Proteomic analysis of grapevine (Vitis vinifera L.) tissues subjected to herbicide stress. J Exp Bot 2783:2795–56
Dubods C, Plomion C (2001) Drought differentially affect expression of a PR10 protein, in needles of maritime pine (Pinus pinaster Ait.) seedlings. J Exp Bot 1143:1144–52
Edreva A (2005) Pathogenesis-related proteins: research progress in the last 15 years. Gen Appl Plant Physiol 31:105–24
Fujimoto Y, Nagata R, Fukasawa H, Yano K, Azuma M, Lida A, Sugimoto S, Shudo K, Hashimoto Y (1998) Purification and cDNA cloning of cytokinin-specific binding protein from mung bean (Vigna radiate). Eur J Biochem 794:802–258
Gonneau M, Pagant S, Brun F, Laloue M (2001) Photoaffinity labeling with the cytokinin agonist azido-CPPUof a 34 kDa peptide of the intracellular pathogenesis-related protein family in the moss physcomitrella patens. Plant Mol Biol 539:548–46
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Bio 463:499–51
Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T (2004) A novel rice PR10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stress, possibly via the jasmonic acid signalling pathway. Plant Cell Physiol 45:550–9. doi:10.1093/pcp/pch063
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. CAB Tech Commun 431:432–22
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 802-:806–81
Hwang HJ, Kim H, Yu HJ, Oh MH, Lee I, Kim SG (2003) Gene encoding pathogenesis-related 10 protein of Lithospermum erythrorhizon is responsive to exogenous stimuli related to the plant defense system. Plant Sci 1297:1302–165. doi:10.1016/s0168-9452(03)00341-8
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 163:168–153
Jacobs AK, Dry IB, Robinson SP (1999) Induction of different pathogenesis-related cDNA in grapevine infected with powdery mildew and treated with ethephon. Plant Pathol 325:336–48
Jain S, Srivastava S, Sarin NB, Kav NN (2006) Proteomics reveals elevated levels of PR 10 proteins in saline-tolerant peanut (Arachis hypogaea) calli. Plant Physiol Biochem. 44:253–9
Jellouli N, Ben Jouira H, Skouri H, Gargouri A, Ghorbel A, Mliki A (2008) Proteomic analysis of Tunisian grapevine cultivar Razegui under salt stress. J Plant Physiol 471:481–165. doi:10.1016/j.jplph.2007.02.009
Kav NNV, Srivastava S, Goonewrdene L, Blade SF (2005) Proteome-level change in the roots of Pisum sativum in response to salinity. Ann Appl Biol 145:217–30. doi:10.1111/j.1744-7348.2004.tb00378.x
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 680:685–22
Liu J, Zhu JK (1998) A calcium sensor homolog required for plant salt tolerance. Science. 280:1943–1945
Mendoza FJ, Quintero RA, Bressan PM, Hasegawa JM, Pardo J (1996) Activated calcineurin confers high tolerance to ion stress and alters budding patterns and cell morphology of yeast cells. J Biol Chem 271:23061–7. doi:10.1074/jbc.271.38.23061
Moon H, Lee B, Choi G, Shin D, Prasad DT, Lee O, Kwak SS, Kim DH, Nam J, Hong JC, Lee SY, Cho MJ, Liim CO, Yun DJ (2003) NDP kinase 2 interacts with tow oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc Natl Acad Sci USA 358:363–100. doi:10.1105/tpc.9.12.2243
Niskanen R, Dris R (2004) Pathogenesis-related proteins—an important group of plant-derived allergens. Food Agric Environ 14:24–2
Okushima Y, Koizumi N, Kusano T, Sano H (2000) Secreted proteins of tobacco cultured BY2 cells: identification of a new member of pathogenesis-related proteins. Plant Mol Biol 42:479–88. doi:10.1023/A:1006393326985
Park CJ, Kim KJ, Shin R, Park JM, Shin YC, Paek KH (2004) Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J 186:198–37. doi:10.1046/j.1365-313X.2003.01951.x
Pnueli L, Hallak-Herr E, Rozenberg M, Cohen M, Goloubinoff P, Kplan A, Mittler R (2002) Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retma raetam. Plant J 319:330–1
Przymusinski R, Rucinska R, Gwozdz EA (2004) Increased accumulation of pathogenesis-related proteins in response of lupine roots to various abiotic stresses. Environ Exp Bot 52:53–61. doi:10.1016/j.envexpbot.2004.01.006
Rakwal R, Agrawal GK, Yonekura M (1999) Separation of proteins from stressed rice (Oryza sativa L.) leaf tissues by two-dimensional polyacrylamide gel electrophoresis: induction of pathogenesis-related and cellular protect proteins by jasmonic acid, UV irradiation and copper chloride. Electrophoresis 3472:3478–20
Renault AS, Deloire A, Letinois I, Kraeva E, Tesniere C, Ageorges A, Redon C, Bierne J (2000) Beta-1, 3-Glucanase gene expression in grapevine leaves as a response to infection with Botrytis cinerea. Am J Enol Vitic 81:87–51
Schuster AM, Davies E (1983) Ribonucleic acid and protein metabolism in pea epicotyls. II. Response to wounding in aged tissue. Plant Physiol 73:817–21. doi:10.1104/pp.73.3.817
Serrano R, Culiañz-Maciá A, Moreno V (1999) Geneticn engineering of salt and drought tolerance with yeast regulatory genes. Sci Hortic (Amsterdam) 78:261–9. doi:10.1016/S0304-4238(98)00196-4
Shalu J, Srivastava S, Neera BSKAVNNV (2006) Proteomics reveals elevated levels of PR 10 proteins in saline-tolerant peanut (Arachis hypogaea) calli. Plant Physiol Biochem 44:253–9. doi:10.1016/j.plaphy.2006.04.006
Somssich IE, Schmelzer E, Kawallek P, Hahlbrock K (1988) Gene structure and in situ transcript localization of pathogenesis-related 1 in parsley. Mol Gen Genet 93:98–213
Srivastava S, Fristensky B, Kav NNV (2004) Constitutive expression of a PR10 protein enhances the germination of Brassica napus under saline conditions. Plant Cell Physiol 1320:1324–45
Trueman LJ. Heterologous Expression in Yeast. Plant Gene Transfer and Expression Protocols 49: 341-354
Van Loon LC. Bakker PA. Pieterse HM. Cornelis MJ. (2006) Prospects and challenges for practical application of rhizobacteria-mediated induced systemic resistance. Article in monograph proceedings Biologie.
Van Loon LC, Pierpoint WS, Boller Th, Conejero V (1994) Recommendations for naming plant pathogenesis-related proteins. Plant Mol Biol Rep 245:264–12
Warner SAJ, Gill A, Draper J (1994) The developmental expression of the asparagus intracellular PR protein (AoPR1) gene correlates with sites of phenylpropanoide biosynthesis. Plant 31:34–6
Wei Y, Zhang Z, Andersen CH, Schmelzer E, Gregersen PL, Collinge DB, Smedegaard-Petersen V, Thordal-Christensen H (1998) An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol Biol 36:101–12. doi:10.1023/A:1005955119326
Zhang Z, Collinge DB, Thordal-Christensen H (1995) Germin-like oxalate oxidase, a H2O2-producing enzyme, accumulates in barley attacked by the powdery mildew fungus. Plant J 8:139–45. doi:10.1046/j.1365-313X.1995.08010139.x
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 247:273–53
Acknowledgments
The authors wish to express their sincere thanks to Lamia Djemal for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jellouli, N., Ben Jouira, H., Daldoul, S. et al. Proteomic and Transcriptomic Analysis of Grapevine PR10 Expression During Salt Stress and Functional Characterization in Yeast. Plant Mol Biol Rep 28, 1–8 (2010). https://doi.org/10.1007/s11105-009-0116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-009-0116-1