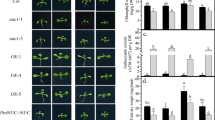
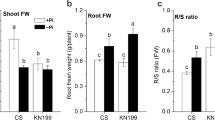
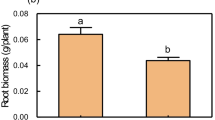
Abstract
Aims
The aims of this work were to investigate phosphate starvation responses of Brassica napus (B. napus) under heterogeneous phosphate (Pi) supply and the regulatory role of jasmonic acid (JA) in the systemic response to Pi starvation.
Methods
A split-root system with two separated compartments was employed to mimic heterogeneous Pi distribution in the soil and to examine the effect of heterogeneous Pi supply, and JA or DIECA (JA biosynthesis inhibitor) on growth, root morphology, Pi concentration, Acid phosphatase (APase) activity, nutrition uptake, JA concentration and expression of Pi starvation systemically-induced (PSSI) genes of B. napus.
Results
Heterogeneous Pi supply systemically modified root morphology that increased the total root surface area (TRSA), total root volume (TRV), total root length (TRL) and total lateral root number (TLRN) of root with local Pi supply (R +) and decreased them of root with local no Pi supply (R-) when compared to root with homogeneous Pi supply (R + +) and root devoid of Pi (R–), respectively. Anthocyanin, APase activity and JA concentration in shoot and root of B. napus were systemically regulated by heterogeneous Pi supply. In addition, heterogeneous Pi supply significantly promoted nutrient uptake when compared with homogeneous no Pi supply. Root morphology of B. napus was significantly changed by exogenous addition of JA or DIECA in a split-root system. JA enhanced Pi starvation response by inducing expression of PSSI genes in shoots and roots.
Conclusions
Our results suggest that JA enhances systemic Pi starvation response of B. napus by regulating root morphology, Pi homeostasis and inducing expression of PSSI genes under heterogeneous Pi supply.








Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online.
Abbreviations
- Pi:
-
Phosphate
- JA:
-
Jasmonic acid
- DIECA:
-
Diethyldithiocarbamic acid
- APase:
-
Acid phosphatase
- PSSI:
-
Pi starvation systemically-induced
- TRSA:
-
Total root surface area
- TRV:
-
Total root volume
- TRL:
-
Total root length
- TLRN:
-
Total lateral root number
- P:
-
Phosphorus
- JA-Ile:
-
Jasmonoyl-L-isoleucine
- PR:
-
Primary root
- LR:
-
Lateral root
- 1°LR:
-
First-order lateral root
- 2°LR:
-
Second-order lateral root
References
Alewell C, Ringeval B, Ballabio C, Robinson DA, Panagos P, Borrelli P (2020) Global phosphorus shortage will be aggravated by soil erosion. Nature Commun 11:4546
Ali MS, Baek K-H (2020) Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int J Mol Sci 21:621
An J, Xu R, Liu X, Zhang J, Wang X, You C, Hao Y (2021) Jasmonate induces biosynthesis of anthocyanin and proanthocyanidin in apple by mediating the JAZ1–TRB1–MYB9 complex. Plant J 106:1414–1430
Baker A, Ceasar SA, Palmer AJ, Paterson JB, Qi W, Muench SP, Baldwin SA (2015) Replace, reuse, recycle: Improving the sustainable use of phosphorus by plants. J Exp Bot 66:3523–3540
Chacón-López A, Ibarra-Laclette E, Sánchez-Calderón L, Gutiérrez-Alanís D, Herrera-Estrella L (2011) Global expression pattern comparison between low phosphorus insensitive 4 and WT Arabidopsis reveals an important role of reactive oxygen species and jasmonic acid in the root tip response to phosphate starvation. Plant Signal Behav 6:382–392
Chen S, Ding G, Wang Z, Cai H, Xu F (2015) Proteomic and comparative genomic analysis reveals adaptability of Brassica napus to phosphorus-deficient stress. J Proteomics 117:106–119
Chien PS, Chiang CP, Leong SJ, Chiou TJ (2018) Sensing and signaling of phosphate starvation: From local to long distance. Plant Cell Physiol 59:1714–1722
Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5:344–350
Guo Q, Major IT, Howe GA (2018) Resolution of growth–defense conflict: mechanistic insights from jasmonate signaling. Annu Rev Plant Biol 44:72–81
Gutiérrez-Alanís D, Ojeda-Rivera JO, Yong-Villalobos L, Cárdenas-Torres L, Herrera-Estrella L (2018) Adaptation to phosphate scarcity: Tips from Arabidopsis roots. Trends Plant Sci 23:721–730
Ham BK, Chen J, Yan Y, Lucas WJ (2018) Insights into plant phosphate sensing and signaling. Curr Opin Plant Biol 49:1–9
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2012) Functions of macronutrients. In: Marschner’s mineral nutrition of higher plants. Elsevier, pp 135–189
He K, Du J, Han X, Li H, Kui M, Zhang J, Huang Z, Fu Q, Jiang Y, Hu Y (2023) PHOSPHATE STARVATION RESPONSE1 (PHR1) interacts with JASMONATE ZIM-DOMAIN (JAZ) and MYC2 to modulate phosphate deficiency-induced jasmonate signaling in Arabidopsis. Plant Cell 35:2132–2156
Howe GA, Major IT, Koo AJ (2018) Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol 69:387–415
Hu S, Yu K, Yan J, Shan X, Xie D (2023) Jasmonate perception: ligand-receptor interaction, regulation and evolution. Mol Plant 16:23–42
Huang H, Liu B, Liu L, Song S (2017) Jasmonate action in plant growth and development. J Exp Bot 68:1349–1359
Jia X, Liu P, Lynch JP (2018) Greater lateral root branching density in maize improves phosphorus acquisition from low phosphorus soil. J Exp Bot 69:4961–4970
Jin K, White PJ, Whalley WR, Shen J, Shi L (2017) Shaping an optimal soil by root–soil interaction. Trends Plant Sci 22:823–829
Jost R, Pharmawati M, Lapis-Gaza HR, Rossig C, Berkowitz O, Lambers H, Finnegan PM (2015) Differentiating phosphate-dependent and phosphate-independent systemic phosphate-starvation response networks in Arabidopsis thaliana through the application of phosphite. J Exp Bot 66:2501–2514
Khan GA, Vogiatzaki E, Glauser G, Poirier Y (2016) Phosphate deficiency induces the jasmonate pathway and enhances resistance to insect herbivory. Plant Physiol 171:632–644
Kirkby EA, Johnston AE (2008) Soil and fertilizer phosphorus in relation to crop nutrition. Ecophysiol Plant-Phosphorus Interact, pp 177–223
Koo AJ (2018) Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress response. Phytochem Rev 17:51–80
Leong SJ, Lu WC, Chiou TJ (2018) Phosphite-mediated suppression of anthocyanin accumulation regulated by mitochondrial ATP synthesis and sugars in arabidopsis. Plant Cell Physiol 59:1158–1169
Li H, Ma Q, Li H, Zhang F, Rengel Z, Shen J (2014) Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant Soil 376:151–163
Li Y, Yang X, Liu H, Wang W, Wang C, Ding G, Xu F, Wang S, Cai H, Hammond JP (2022) Local and systemic responses conferring acclimation of Brassica napus roots to low phosphorus conditions. J Exp Bot 73:4753–4777
Liang C, Sun L, Yao Z, Liao H, Tian J (2012) Comparative analysis of PvPAP gene family and their functions in response to phosphorus deficiency in common bean. PLoS ONE 7:16–20
Liu H, Li X, Xiao J, Wang S (2012) A convenient method for simultaneous quantification of multiple phytohormones and metabolites: Application in study of rice-bacterium interaction. Plant Methods 8:2
Liu Q, Zhou GQ, Xu F, Yan XL, Liao H, Wang JX (2013) The involvement of auxin in root architecture plasticity in Arabidopsis induced by heterogeneous phosphorus availability. Biol Plantarum 57:739–748
López-Arredondo DL, Leyva-González MA, González-Morales SI, López-Bucio J, Herrera-Estrella L (2014) Phosphate nutrition: improving low-phosphate tolerance in crops. Annu Rev Plant Biol 65:95–123
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol 156:1041–1049
Lynch JP, Wojciechowski T (2015) Opportunities and challenges in the subsoil: pathways to deeper rooted crops. J Exp Bot 66:2199–2210
Maillard A, Etienne P, Diquélou S, Trouverie J, Billard V, Yvin JC, Ourry A (2016) Nutrient deficiencies modify the ionomic composition of plant tissues: A focus on cross-talk between molybdenum and other nutrients in Brassica napus. J Exp Bot 67:5631–5641
Oldroyd GE, Leyser O (2020) A plant’s diet, surviving in a variable nutrient environment. Science 368:eaba0196
Pandey BK, Verma L, Prusty A, Singh AP, Bennett MJ, Tyagi AK, Giri J, Mehra P (2021) OsJAZ11 regulates phosphate starvation responses in rice. Planta 254:8
Paz-Ares J, Puga MI, Rojas-Triana M, Martinez-Hevia I, Diaz S, Poza-Carrión C, Miñambres M, Leyva A (2022) Plant adaptation to low phosphorus availability: Core signaling, crosstalks and applied implications. Mol Plant 15:104–124
Péret B, Clément M, Nussaume L, Desnos T (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16:442–450
Puga MI, Rojas-Triana M, de Lorenzo L, Leyva A, Rubio V, Paz-Ares J (2017) Novel signals in the regulation of Pi starvation responses in plants: facts and promises. Annu Rev Plant Biol 39:40–49
Ren F, Zhao C-Z, Liu C-S, Huang K-L, Guo Q-Q, Chang L-L, Xiong H, Li X-B (2014) A Brassica napus PHT1 phosphate transporter, BnPht1; 4, promotes phosphate uptake and affects roots architecture of transgenic Arabidopsis. Plant Mol Biol 86:595–607
Scheible W, Rojas-Triana M (2015) Sensing, signalling, and control of phosphate starvation in plants: molecular players and applications. Annu Plant Rev 48:23–63
Shen J, Li H, Neumann G, Zhang F (2005) Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Sci 168:837–845
Sun B, Gao Y, Lynch J (2018) (2018) Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol 177:00234
Takahashi F, Shinozaki K (2019) Long-distance signaling in plant stress response. Annu Rev Plant Biol 47:106–111
Tao Y, Huang J, Jing HK, Shen RF, Zhu XF (2022) Jasmonic acid is involved in root cell wall phosphorus remobilization through the nitric oxide dependent pathway in rice. J Exp Bot 73:2618–2630
Thibaud MC, Arrighi JF, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L (2010) Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J 64:775–789
Ticconi CA, Delatorre CA, Abel S (2001) Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiol 127:963–972
Wan S, Xin X-F (2022) Regulation and integration of plant jasmonate signaling: a comparative view of monocot and dicot. J Genet Genomics 49:704–714
Wang L, Li Z, Qian W et al (2011) The Arabidopsis purple acid phosphatase AtPAP10 is predominantly associated with the root surface and plays an important role in plant tolerance to phosphate limitation. Plant Physiol 157:1283–1299
Wang C, Huang W, Ying Y, Li S, Secco D, Tyerman S, Whelan J, Shou H (2012) Functional characterization of the rice SPX-MFS family reveals a key role of OsSPX-MFS1 in controlling phosphate homeostasis in leaves. New Phytol 196:139–148
Wang X, Feng J, White PJ, Shen J, Cheng L (2019) Heterogeneous phosphate supply influences maize lateral root proliferation by regulating auxin redistribution. Ann Bot 125:119–130
White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM (2013) Matching roots to their environment. Ann Bot 112:207–222
Yan J, Yao R, Chen L, Li S, Gu M, Nan F, Xie D (2018) Dynamic perception of jasmonates by the F-box protein COI1. Mol Plant 11:1237–1247
Acknowledgements
We thank Dr. Feng Ren (Central China Normal University, Wuhan, China) for kindly providing us with seeds of transgenic A. thaliana pBnPht1;4-GUS. This work was supported by the National Nature Science Foundation of China (Grant No. 31972498 and 32172662). Sergey Shabala acknowledges a support from the Australian Research Council (project LP200200132).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Mian Gu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 24 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Yang, X., Li, X. et al. Jasmonic acid participating in the systemic regulation of phosphate starvation response in Brassica napus. Plant Soil (2023). https://doi.org/10.1007/s11104-023-06355-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-06355-2