Abstract
Background and aims
The introduced lineage of Phragmites australis (haplotype M) in North America outcompetes the native lineage (haplotype E). Previous studies have found that haplotype M is situated on the fast side of the trait economic spectrum compared to haplotype E in North America and Europe. The present study evaluated the plant traits and associated soil microbes of introduced and native Phragmites lineages using a common garden experiment.
Methods
Four geographic groups, including the introduced lineage of North America (NAint, haplotype M), native lineage of North America (NAnat, haplotype E), European group (EU, haplotype M) and northwestern China group (CHN, haplotype M), were studied in two life-history stages – growing period and withering period.
Results
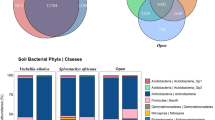
No trait differences were observed among the four groups. The difference existed in the soil microbial structure. The soils derived from the four groups had different bacterial generic structures. NAnat gathered more plant pathogenic and ectomycorrhizal fungi in the growing period. In the withering period, NAint accumulated more plant pathogenic fungi, while NAnat accumulated more arbuscular mycorrhizal fungi. The soil microbial structure was different among NAint, EU and CHN despite the same haplotype.
Conclusion
The interaction between plant traits and the soil microbes seemed weak. However, the long-term effects of microbial transition on the introduced and native lineages are unknown, and the potential plant-soil interactions need further exploration.









Similar content being viewed by others
Data Availability
Data will be made available on reasonable request.
References
Albert A, Brisson J, Belzile F, Turgeon J, Lavoie C (2015) Strategies for a successful plant invasion: the reproduction of Phragmites australis in north-eastern North America. J Ecol 103:1529–1537
An JX, Wang Q, Yang J, Liu JQ (2012) Phylogeographic analyses of Phragmites australis in China: native distribution and habitat preference of the haplotype that invaded North America. J Syst Evol 50:334–340
Aßhauer KP, Wemheuer B, Daniel R, Meinicke P (2015) Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31:2882–2884
Baldrian P, Kohout P (2017) Interactions of saprotrophic fungi with tree roots: can we observe the emergence of novel ectomycorrhizal fungi? New Phytol 215:511–513
Bevivino A, Dalmastri C (2017) Chap. 5: impact of agricultural land management on soil bacterial community: a case study in the Mediterranean area. In: Lukac M, Grenni P, Gamboni M (eds) Soil biological communities and ecosystem resilience. Springer International Publishing AG, Gewerbestrasse, Cham, Switzerland, pp 77–95
Bickford WA, Goldberg DE, Kowalski K, Zak DR (2018) Root endophytes and invasiveness: no difference between native and non-native Phragmites in the Great Lakes Region. Ecosphere 9:e02526
Bickford WA, Goldberg DE, Zak DR, Snow DS, Kowalski KP (2022) Plant effects on and response to soil microbes in native and non-native Phragmites australis. Ecol Appl 32:e2565
Bickford WA, Zak DR, Kowalski KP, Goldberg DE (2020) Differences in rhizosphere microbial communities between native and non-native Phragmites australis may depend on stand density. Ecol Evol 10:11739–11751
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733
Caplan JS, Wheaton CN, Mozdzer TJ (2014) Belowground advantages in construction cost facilitate a cryptic plant invasion. AoB Plants 6:plu020
Davet P (2004) Chap. 7: interactions between microorganisms and plants. In: Davet P (ed) Microbial ecology of the soil and plant growth. Taylor & Francis Group, Boca Raton, US, pp 189–268
De Long JR, Heinen R, Hannula SE, Jongen R, Steinauer K, Bezemer TM (2023) Plant-litter-soil feedbacks in common grass species are slightly negative and only marginally modified by litter exposed to insect herbivory. Plant Soil 485:227–244
DeGasparro SL, Beresford DV, Prater C, Frost PC (2020) Leaf litter decomposition in boreal lakes: variable mass loss and nutrient release ratios across a geographic gradient. Hydrobiologia 847:819–830
Dong N, Prentice IC, Wright IJ, Wang H, Atkin OK, Bloomfield KJ, Domingues TF, Gleason SM, Maire V, Onoda Y, Poorter H, Smith NG (2022) Leaf nitrogen from the perspective of optimal plant function. J Ecol 110:2585–2602
Elgersma KJ (2014) Soils suppressing and promoting non-native plant invasions. In: Dighton J, Krumins JA (eds) Interactions in soil: promoting plant growth. Springer Science + Business Media, Dordrecht, pp 181–202
Eppinga MB, Rietkerk M, Dekker SC, De Ruiter PC, van der Putten WH, Eppinga MB, Rietkerk M, Dekker SC, De Ruiter PC, Van der Putten WH (2006) Accumulation of local pathogens: a new hypothesis to explain exotic plant invasions. Oikos 114:168–176
Farrer EC, Birnbaum C, Waryszak P, Halbrook SR, Brady MV, Bumby CR, Candaele H, Kulick NK, Lee SFH, Schroeder CS, Smith MKH, Wilber W (2021) Plant and microbial impacts of an invasive species vary across an environmental gradient. J Ecol 109:2163–2176
Frevola DM, Hovick SM (2019) The independent effects of nutrient enrichment and pulsed nutrient delivery on a common wetland invader and its native conspecific. Oecologia 191:447–460
Gao GF, Li PF, Zhong JX, Shen ZJ, Chen J, Li YT, Isabwe A, Zhu XY, Ding QS, Zhang S, Gao CH, Zheng HL (2019) Spartina alterniflora invasion alters soil bacterial communities and enhances soil N2O emissions by stimulating soil denitrification in mangrove wetland. Sci Total Environ 653:231240
Guo WY, Lambertini C, Nguyen LX, Li XZ, Brix H (2014) Preadaptation and post-introduction evolution facilitate the invasion of Phragmites australis in North America. Ecol Evol 4:4567–4577
Hatfield JL, Dold C (2019) Water-use efficiency: advances and challenges in a changing climate. Front Plant Sci 10:103
Inderjit, van der Putten WH (2010) Impacts of soil microbial communities on exotic plant invasions. Trends Ecol Evol 25:512–519
Ingold CT, Hudson HJ (1993) Ecology of saprotrophic fungi. In: Ingold CT, Hudson HJ (eds) The biology of fungi. Springer, Dordrecht, pp 145–157
Jin KM, White PJ, Whalley WR, Shen JB, Shi L (2017) Shaping an optimal soil by root-soil interaction. Trends Plant Sci 22:823–829
Katul GG, Ellsworth DS, Lai CT (2000) Modelling assimilation and intercellular CO2 from measured conductance: a synthesis of approaches. Plant Cell Environ 23:1313–1328
Lau JA, Lennon JT (2011) Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection on plant traits. New Phytol 192:215–224
Lau JA, Suwa T (2016) The changing nature of plant–microbe interactions during a biological invasion. Biol Invasions 18:3527–3534
Leishman MR, Haslehurst T, Ares A, Baruch Z (2007) Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol 176:635–643
Li YP, Li HB, Li YY, Zhang SQ (2017) Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J 5:231–239
Liao HX, Pal RW, Niinemets Ü, Bahn M, Cerabolini BEL, Peng SL (2021) Different functional characteristics can explain different dimensions of plant invasion success. J Ecol 109:1524–1536
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Mangla S, Inderjit, Callaway RM (2008) Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J Ecol 96:58–67
McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am J Bot 89:1431–1438
Meyerson LA, Cronin JT, Bhattarai GP, Brix H, Lambertini C, Lučanová M, Rinehart S, Suda J, Pyšek P (2016) Do ploidy level and nuclear genome size and latitude of origin modify the expression of Phragmites australis traits and interactions with herbivores? Biol Invasions 18:2531–2549
Meyerson LA, Cronin JT, Pyšek P (2016) Phragmites australis as a model organism for studying plant invasions. Biol Invasions 18:2421–2431
Mommer L, Weemstra M (2012) The role of roots in the resource economics spectrum. New Phytol 195:725–727
Montesinos D (2022) Fast invasives fastly become faster: invasive plants align largely with the fast side of the plant economics spectrum. J Ecol 110:1010–1014
Mozdzer TJ, Brisson J, Hazelton ELG (2013) Physiological ecology and functional traits of north american native and eurasian introduced Phragmites australis lineages. AoB Plants 5:plt048–plt048
Mozdzer TJ, Zieman JC (2010) Ecophysiological differences between genetic lineages facilitate the invasion of non-native Phragmites australis in North American Atlantic coast wetlands. J Ecol 98:451–458
Nguyen NH, Song ZW, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Oh DH, Kowalski KP, Quach QN, Wijesinghege C, Tanford P, Dassanayake M, Clay K (2022) Novel genome characteristics contribute to the invasiveness of Phragmites australis (common reed). Mol Ecol 31:1142–1159
Packer JG, Meyerson LA, Skálová H, Pyšek P, Kueffer C (2017) Biological Flora of the british Isles: Phragmites australis. J Ecol 105:1123–1162
Palma E, Vesk PA, White M, Baumgartner JB, Catford JA (2021) Plant functional traits reflect different dimensions of species invasiveness. Ecology 102:e03317
Pérez-Harguindeguy N, Díaz S, Garnier E, Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234
Plut K, Paul J, Ciotir C, Major M, Freeland JR (2011) Origin of non-native Phragmites australis in North America, a common wetland invader. Fund Appl Limnol 179:121–129
Pyšek P, Skálová H, Čuda J, Guo WY, Doležal J, Kauzál O, Lambertini C, Pyšková K, Brix H, Meyerson LA (2019) Physiology of a plant invasion: biomass production, growth and tissue chemistry of invasive and native Phragmites australis populations. Preslia 91:51–75
Ravichandran KR, Thangavelu M (2017) Role and influence of soil microbial communities on plant invasion. Ecol Quest 3:9–23
Ren LJ, Guo X, Liu SN, Yu T, Guo WH, Wang RQ, Ye SY, Lambertini C, Brix H, Eller F (2020) Intraspecific variation in Phragmites australis: clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. J Ecol 108:2531–2543
Rengel Z (2002) Genetic control of root exudation. Plant Soil 245:59–70
Roumet C, Birouste M, Picon-Cochard C, Ghestem M, Osman N, Vrignon-Brenas S, Cao KF, Stokes A (2016) Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytol 210:815–826
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. P Natl Acad Sci USA 99:2445–2449
Schroeder CS, Halbrook S, Birnbaum C, Waryszak P, Wilber W, Farrer EC (2022) Phragmites australis associates with belowground fungal communities characterized by high diversity and pathogen abundance. Diversity 12:363
van der Heijden MGA, Martin FM, Selosse M, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423
Wang T, Dou HR, Liu CH, Yu D (2021) Decoupling between plant growth and functional traits of the free-floating fern Salvinia natans under shifted water nutrient stoichiometric regimes. Flora 281:151876
Wang T, Hu JY, Wang RQ, Liu CH, Yu D (2019) Trait convergence and niche differentiation of two exotic invasive free-floating plant species in China under shifted water nutrient stoichiometric regimes. Environ Sci Pollut Res 26:35779–35786
Wang CY, Zhou JW, Liu J, Jiang K, Xiao HG, Du DL (2018) Responses of the soil fungal communities to the co-invasion of two invasive species with different cover classes. Plant Biol 20:151–159
Wen ZH, White PJ, Shen JB, Lambers H (2022) Linking root exudation to belowground economic traits for resource acquisition. New Phytol 233:1620–1635
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Xue W, Huang L, Yu FH, Bezemer TM (2018) Intraspecific aggregation and soil heterogeneity: competitive interactions of two clonal plants with contrasting spatial architecture. Plant Soil 425:231–240
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loqué D, Bowen BP, Firestone MK, Northen TR, Brodie EL (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480
Zhang MH, Cheng XL, Geng QH, Shi Z, Luo YQ, Xu X (2019a) Leaf litter traits predominantly control litter decomposition in streams worldwide. Global Ecol Biogeogr 28:1469–1486
Zhang P, Li B, Wu JH, Hu SJ (2019b) Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol Lett 22:200–210
Zhang HY, Goncalves P, Copeland E, Qia SS, Dai ZC, Li GL, Wang CY, Du DL, Thomas T (2020) Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol Biochem 143:107739
Acknowledgements
We deeply thank Novogene Co., Ltd. for soil microbial analyses and Nanjing Institute of Geography & Limnology, Chinese Academy of Sciences for elemental analyses. The present research was financially supported by the National Natural Science Foundation of China (31800299, 32271588), the Qingdao Agricultural University Doctoral Start-Up Fund (663/1121009) and the National Undergraduate Training Program for Innovation and Entrepreneurship of Qingdao Agricultural University (844, 849).
Author information
Authors and Affiliations
Contributions
T. W. and X. G. proposed the idea, designed the experiment and wrote the manuscript. J. Y. performed the visualization. X. C., Y. Z., X. H. and H. D performed the sampling and data collection. Z. W. analyzed the data. R. W. provided the experimental infrastructure and facilities and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Jonathan Richard De Long.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Guo, X., Yang, J. et al. The introduced lineage of Phragmites australis in North America differs from its co-existing native lineage in associated soil microbial structure rather than plant traits. Plant Soil 493, 137–156 (2023). https://doi.org/10.1007/s11104-023-06216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06216-y