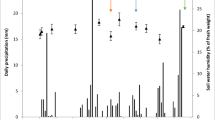
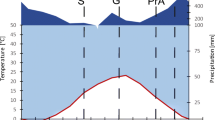
Abstract
We quantified the distribution of nitrogen (N), dry-matter (biomass) and of soil-applied 15 N in tree and soil compartments in five naturally-growing 20-year-old oak trees. After applying 15 N solution to soil at the base of the trees in spring, all the trees were felled in the fall, their root system excavated, biomass, nitrogen and 15 N content measured in all compartments. Xylem rings-compartment contains most biomass (47%) while branches and coarse-root contains most nitrogen (29% and 14% respectively). The labelled 15 N absorbed throughout the vegetation season, was found in all compartments except the heartwood. The majority of recovered 15 N was in the leaves (24%). Some often overlooked compartments (coarse root, stump, xylem and other branches) together recovered 45% of the 15 N. 15 N was found in all the sapwood rings, from the ring formed in the current year up to 10 year-old rings, marking the limit of the heartwood. More 15 N was found in the younger rings compared to older rings. The 15 N allocated to ancient rings can originate from different, non-mutually exclusive, sources: whether directly from the soil via the 15 N uptake throughout the vegetation season and transport in the xylem sap, from the autumnal resorption of 15 N first allocated to the leaves, or from the 15 N mobility once allocated to the forming ring to older rings through ray parenchyma. With about 6% of the initial 15 N retrieved in the microbial biomass at the end of the growing season, we confirmed the role of microbial biomass as forest nitrogen sink.







Similar content being viewed by others
Abbreviations
- DBH:
-
Diameter at Breast Height
- DOY:
-
Day of the Year
- Stump:
-
The tree's pivot that is inside the soil
- AG:
-
Aboveground
- BG:
-
Belowground
- EP:
-
Experimental plot
References
Aerts R (1996) Nutrient resorption from senescing leaves of perennials: are there general patterns? J Ecol 597–608
Amponsah IG, Lieffers VJ, Comeau PG, Landhäusser SM (2004) Nitrogen-15 uptake by Pinus contorta seedlings in relation to phenological stage and season. Scand J For Res 19(4):329–338
Babst BA, Coleman GD (2018) Seasonal nitrogen cycling in temperate trees: transport and regulatory mechanisms are key missing links. Plant Sci 270:268–277
Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157(3):605–615. https://doi.org/10.1046/j.1469-8137.2003.00681.x
Bazot S, Barthes L, Blanot D, Fresneau C (2013) Distribution of non-structural nitrogen and carbohydrate compounds in mature oak trees in a temperate forest at four key phenological stages. Trees 27(4):1023–1034
Bazot S, Fresneau C, Damesin C, Barthes L (2016) Contribution of previous year’s leaf N and soil N uptake to current year’s leaf growth in sessile oak. Biogeosciences 13(11):3475–3484. https://doi.org/10.5194/bg-13-3475-2016
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Brown S, Gillespie A, Lugo AE (1989) Biomass estimation methods for tropical forests with applications to forest inventory data. For Sci 35:881–902
Čermák J, Cienciala E, Kučera J, Hällgren JE (1992) Radial velocity profiles of water flow in trunks of Norway spruce and oak and the response of spruce to severing. Tree Physiol 10(4):367–380
Chave J, Rie’ra B, Dubois MA (2001) Estimation of biomass in a neotropical forest of French Guiana: spatial and temporal variability. J Trop Ecol 17:79–96
Chave J, Réjou-Méchain M, Búrquez A, Chidumayo E, Colgan MS, Vieilledent DWB, G, et al (2014) Improved allometric models to estimate the aboveground biomass of tropical trees. Glob Change Biol 20(10):3177–3190
Colin-Belgrand M, Ranger J, Bouchon J (1996) Internal nutrient translocation in chestnut tree stemwood: III. Dynamics across an age series of Castanea sativa (Miller). Ann Bot 78(6):729–740
Cooke JEK, Weih M (2005) Nitrogen storage and seasonal nitrogen cycling in Populus: Bridging molecular physiology and ecophysiology. New Phytol 167(1):19–30. https://doi.org/10.1111/j.1469-8137.2005.01451.x
Crow TR (1978) Common regressions to estimate tree biomass in tropical stands. For Sci 24:110–114
Cunia T (1987) Error of forest inventory estimates: its main components. In: Wharton EH, Cunia T (eds) Estimating tree biomass regressions and their error. USDA For Serv Gen Tech Rep NE-117, p 303
Deléens E, Morot-Gaudry J, Martin F, Thoreux A, Gojon A (1997) Méthodologie 15N. In: Morot-Gaudry J (ed) Assimilation de l’azote chez les plantes Aspects physiologique, biochimique et moléculaire. INRA, Paris, pp 265–280
Delpierre N, Berveiller D, Granda E, Dufrêne E (2016) Wood phenology, not carbon input, controls the interannual variability of wood growth in a temperate oak forest. New Phytol 210(2):459–470
Dickson RE (1989) Carbon and nitrogen allocation in trees. In Annales des sciences forestières (Vol. 46, No. Supplement, pp 631s-647s). EDP Sciences
Drexhage M, Colin F (2001) Estimating root system biomass from breast-height diameters. Forestry 74(5):491–497
Du B, Ji H, Liu S, Kang H, Yin S, Liu C (2021) Nutrient resorption strategies of three oak tree species in response to interannual climate variability. Forest Ecosystems 8(1):1–11
Eastman BA, Adams MB, Brzostek ER, Burnham MB, Carrara JE, Kelly C, McNeil BE, Walter CA, Peterjohn WT (2021) Altered plant carbon partitioning enhanced forest ecosystem carbon storage after 25 years of nitrogen additions. New Phytol 230:1435–1448. https://doi.org/10.1111/nph.17256
El Zein R, Bréda N, Gérant D, Zeller B, Maillard P (2011a) Nitrogen sources for current-year shoot growth in 50-year-old sessile oak trees: An in situ 15N labeling approach. Tree Physiol 31(12):1390–1400. https://doi.org/10.1093/treephys/tpr118
El Zein R, Maillard P, Bréda N, Marchand J, Montpied P, Gérant D (2011b) Seasonal changes of C and N non-structural compounds in the stem sapwood of adult sessile oak and beech trees. Tree Physiol 31(8):843–854
Elhani S, Lema BF, Zeller B, Bréchet C, Guehl JM, Dupouey JL (2003) Inter-annual mobility of nitrogen between beech rings: a labelling experiment. Ann For Sci 60(6):503–508
Fernández-Martínez M, Vicca S, Janssens IA, Sardans J, Luyssaert S, Campioli M, Peñuelas J (2014) Nutrient availability as the key regulator of global forest carbon balance. Nat Clim Chang 4(6):471–476
Finzi AC, Berthrong ST (2005) The uptake of amino acids by microbes and trees in three cold-temperate forests. Ecology 86(12):3345–3353
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Change Biol 21(5):2082–2094. https://doi.org/10.1111/gcb.12816
Franklin O, Johansson J, Dewar R, Dieckmann U, Mcmurtrie R, Brännström Å, Dybzinski R (2012) Modeling carbon allocation in trees: A search for principles. Tree Physiol 32:648–666. https://doi.org/10.1093/treephys/tpr138
Geßler A, Weber P, Schneider S, Rennenberg H (2003) Bidirectional exchange of amino compounds between phloem and xylem during long-distance transport in Norway spruce trees (Picea abies [L] Karst). J Exp Bot 54(386):1389–1397
Goodale CL, Apps MJ, Birdsey RA, Field CB, Heath LS, Houghton RA, Jenkins JC, Kohlmaier GH, Kurz W, Liu S, Nabuurs GJ, Nilsson S, Shvidenko AZ (2002) Forest carbon sinks in the northern hemisphere. Ecol Appl 12(3):891–899. https://doi.org/10.1890/1051-0761(2002)012[0891:FCSITN]2.0.CO;2
Granier A, Anfodillo T, Sabatti M, Cochard H, Dreyer E, Tomasi M, Bréda N (1994) Axial and radial water flow in the trunks of oak trees: a quantitative and qualitative analysis. Tree Physiol 14(12):1383–1396
Grassi G, House J, Dentener F, Federici S, den Elzen M, Penman J (2017) The key role of forests in meeting climate targets requires science for credible mitigation. Nat Clim Chang 7(3):220–226. https://doi.org/10.1038/nclimate3227
Härdtle W, Niemeyer T, Fichtner A, Li Y, Ries C, Schuldt A, von Oheimb G (2014) Climate imprints on tree-ring δ15N signatures of sessile oak (Quercus petraea Liebl.) on soils with contrasting water availability. Ecol Ind 45:45–50
Hart SC, Classen AT (2003) Potential for assessing long-term dynamics in soil nitrogen availability from variations in δ 15N of tree rings. Isot Environ Health Stud 39(1):15–28. https://doi.org/10.1080/1025601031000102206
Helmisaari HS, Makkonen K, Kellomäki S, Valtonen E, Mälkönen E (2002) Below-and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. For Ecol Manage 165(1–3):317–326
Hendrick RL, Pregitzer KS (1993) The dynamics of fine root length, biomass, and nitrogen content in two northern hardwood ecosystems. Can J For Res 23(12):2507–2520. https://doi.org/10.1139/x93-312
Houghton RA, Lawrence KL, Hackler JL, Brown S (2001) The spatial distribution of forest biomass in the Brazilian Amazon: a comparison of estimates. Glob Change Biol 7:731–746
IUSS Working Group WRB-FAO (2015) IUSS Working Group WRB. 2015. World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports Nº. 106
Jordan MO, Wendler R, Millard P (2012) Autumnal N storage determines the spring growth, N uptake and N internal cycling of young peach trees. Trees 26(2):393–404
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89(2):371–379
Legaz F, Serna MD, Primo-Millo E (1995) Mobilization of the reserve N in citrus. Plant Soil 173(2):205–210. https://doi.org/10.1007/BF00011457
Maxwell TL, Bazot S, Marmagne A, Pinek L, Laffont B, Vincent G, Barthes L (2020) In situ fate of mineral N in the tree-soil-microorganism system before and after budburst in 20-year-old Quercus petraea (Matt.) Liebl. Plant and Soil 455(1):425–438. https://doi.org/10.1007/s11104-020-04610-4
McLauchlan KK, Craine JM (2011) Species-specific trajectories of nitrogen isotopes in Indiana hardwood forests, USA. Biogeosci Discuss 8(3).
Meerts P (2002) Mineral nutrient concentrations in sapwood and heartwood: a literature review. Ann For Sci 59(7):713–722
Migita C, Chiba Y, Tange T (2007) Seasonal and spatial variations in leaf nitrogen content and resorption in a Quercus serrata canopy. Tree Physiol 27(1):63–70
Millard P (1995) Internal cycling of nitrogen in trees. Acta Horticulturae 383:3–14. https://doi.org/10.17660/ActaHortic.1995.383.1
Millard P, Grelet G (2010) Nitrogen storage and remobilization by trees: Ecophysiological relevance in a changing world. Tree Physiol 30(9):1083–1095. https://doi.org/10.1093/treephys/tpq042
Návar J (2009) Allometric equations for tree species and carbon stocks for forests of northwestern Mexico. For Ecol Manage 257(2):427–434
Norby RJ, Warren JM, Iversen CM, Medlyn BE, Mc Murtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci 107(45):19368–19373. https://doi.org/10.1073/pnas.1006463107
Pfautsch S, Renard J, Tjoelker MG, Salih A (2015) Phloem as capacitor: radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. Plant Physiol 167(3):963–971
Plavcová L, Jansen S (2015) The role of xylem parenchyma in the storage and utilization of nonstructural carbohydrates. In Functional and ecological xylem anatomy (pp. 209–234). Springer, Cham
Poulson SR, Chamberlain CP, Friedland AJ (1995) Nitrogen isotope variation of tree rings as a potential indicator of environmental change. Chem Geol 125(3–4):307–315
Quartieri M, Millard P, Tagliavini M (2002) Storage and remobilisation of nitrogen by pear (Pyrus communis L.) trees as affected by timing of N supply. Eur J Agron 17(2):105–110. https://doi.org/10.1016/S1161-0301(01)00141-1
Rennenberg H, Dannenmann M, Gessler A, Kreuzwieser J, Simon J, Papen H (2009) Nitrogen balance in forest soils: nutritional limitation of plants under climate change stresses. Plant Biol 11:4–23
Rennenberg H, Dannenmann M (2015) Nitrogen Nutrition of Trees in Temperate Forests—The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests 6(8). https://doi.org/10.3390/f6082820
Roccuzzo G, Scandellari F, Allegra M, Torrisi B, Stagno F, Mimmo T, Zanotelli D, Gioacchini P, Millard P, Tagliavini M (2017) Seasonal dynamics of root uptake and spring remobilisation of nitrogen in field grown orange trees. Sci Hortic 226:223–230. https://doi.org/10.1016/j.scienta.2017.08.010
Sample R, Babst BA (2019) Timing of nitrogen resorption-related processes during fall senescence in Southern Oak species. For Sci 65(3):245–249
Tadashi K, Sumiharu K, Bambang S (1982) The distribution of the winter absorption element (15N) and the redistribution of the spring. J Acad Sci 50(4):421–426. https://doi.org/10.2503/jjshs.50.421
Ter-Mikaelian MT, Korzukhin MD (1997) Biomass equation for sixty-five North American tree species. For Manag 97:1–24
Tharammal T, Bala G, Narayanappa D, Nemani R (2019) Potential roles of CO2 fertilization, nitrogen deposition, climate change, and land use and land cover change on the global terrestrial carbon uptake in the twenty-first century. Clim Dyn 52(7):4393–4406
Timbal J, Aussenac G (1996) An overview of ecology and silviculture of indigenous oaks in France. Ann For Sci 53(2–3):649–661. https://doi.org/10.1051/forest:19960243
Tognetti R, Raschi A, Béres C, Fenyvesi A, Ridder HW (1996) Comparison of sap flow, cavitation and water status of Quercus petraea and Quercus cerris trees with special reference to computer tomography. Plant Cell Environ 19(8):928–938
Ueyama M, Ichii K, Kobayashi H, Kumagai TO, Beringer J, Merbold L, Yasuda Y (2020) Inferring CO2 fertilization effect based on global monitoring land-atmosphere exchange with a theoretical model. Environ Res Lett 15(8):084009
Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB (2012) Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol Monogr 82(2):205–220
Verheyden A, Kairo JG, Beeckman H, Koedam N (2004) Growth rings, growth ring formation and age determination in the mangrove Rhizophora mucronata. Ann Bot 94(1):59–66. https://doi.org/10.1093/aob/mch115
Vicca S, Luyssaert S, Peñuelas J, Campioli M, Chapin FS III, Ciais P, Janssens IA (2012) Fertile forests produce biomass more efficiently. Ecol Lett 15(6):520–526
Wang C (2006) Biomass allometric equations for 10 co-occurring tree species in Chinese temperate forests. For Ecol Manage 222:9–16. https://doi.org/10.1016/j.foreco.2005.10.074
Xiao CW, Yuste JC, Janssens IA, Roskams P, Nachtergale L, Carrara A, Ceulemans R (2003) Above-and belowground biomass and net primary production in a 73-year-old Scots pine forest. Tree Physiol 23(8):505–516
Yang B, Xue W, Yu S, Zhou J, Zhang W (2019) Effects of stand age on biomass allocation and allometry of quercus acutissima in the Central Loess Plateau of China. Forests 10(1):41
Zeller B, Colin-Belgrand M, Dambrine E, Martin F (2001) Fate of nitrogen released from 15N-labeled litter in European beech forests. Tree Physiol 21(2–3):153–162. https://doi.org/10.1093/treephys/21.2-3.153
Zianis D, Muukkonen P, Mäkipää R, Mencuccini M (2005) Biomass and stem volume equations for tree species in Europe. Silva Fennica Monographs 4:1–63
Acknowledgements
Financial support was provided by Laboratoire Ecologie, Systématique et Evolution and the Institut Diversité Ecologie et Evolution du Vivant through the financing of the TIP-TOP project. The authors would like to acknowledge the contributions of Caroline Mauve, Marlène Sibold-Lamothe, Françoise Gilard, Gregory Mouille for their contribution in the TIP-TOP project, as well as Daniel Berveiller, Patricia Le Thuault, Michèle Viel, Lionel Saunois, Sandrine Fontaine, Alain Sévéré, Liliana Pinek and Baptiste Laffont for data collection, field work, and technical assistance. We are grateful to the French National Forest Office (ONF) for allowing us to carry out these experiments at the Fontainebleau-Barbeau forest. This work benefited from the French state aid managed by the ANR under the "Investissements d'avenir" programme with the reference ANR-16-CONV-0003 through the financial support provided by CLAND to Mubarak MAHMUD during the period of writing this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Xinhua He.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmud, M., Maxwell, T.L., Cueff, S. et al. Recently absorbed nitrogen incorporates into new and old tissues: evidence from a 15 N-labelling experiment in deciduous oaks. Plant Soil 480, 407–421 (2022). https://doi.org/10.1007/s11104-022-05589-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05589-w