Abstract
Background and aims
Competitive vegetation in forest stands influence seedling growth by changing soil nutrient availability. However, studies on the effects of different weed control methods on seedling growth of Chinese fir (Cunninghamia lanceolata (Lamb.) Hook.) are rare.
Methods
We applied three weed control methods, comprising artificial sickle weeding (ASW), woody disc weeding (WDW), and nonwoven cloth weeding (nWCW), to explore their effect on growth of Chinese fir seedlings in a plantation in Jiangxi Province, China.
Results
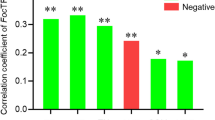
The weed control methods affected the shoot height and root-collar diameter of the seedlings. The contents of sugar, glucose, triglycerides, total cholesterol, and free fatty acids in newly developed leaves were increased after ASW and nWCW treatment, and were consistent with the expression of genes associated with glucokinase, sucrose phosphate synthase, and sucrose synthase. Weeding method influenced soil properties, including pH, moisture, total nitrogen (TN), ammonium-N, nitrate-N, total phosphorus, available phosphorus, and dissolved organic carbon contents. Moisture content was the main factor that influenced the soil bacterial community and leaf nutrition. High-throughput sequencing of the bacterial 16 S rRNA gene revealed that the weeding methods affected bacterial community structure. Specifically, compared with ASW and nWCW, WDW contributed to lower soil bacterial diversity, simpler bacterial interaction, and increase in pathogenic bacteria potential.
Conclusions
The weeding methods differ in influence on soil bacterial community structure, soil properties, and plant growth, which are potentially useful to improve the growth of Chinese fir seedlings. ASW and nWCW strategies were recommended to be applied in the practice of weed control on seedling growth of Chinese fir.











Similar content being viewed by others
References
Ai C, Liang G, Sun J, Wang X, He P, Zhou W, He X (2015) Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol Biochem 80:70–78. https://doi.org/10.1016/j.soilbio.2014.09.028
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell Scientific Publications
Appleton BL, Hill DB (1997) Kentucky Christmas tree production workbook: Vegetation control. University of Kentucky Cooperative Extension Service. Available online: http://www2.ca.uky.edu/agcomm/pubs/for/for23/for23.pdf
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8:1–10. https://doi.org/10.1111/j.1462-2920.2005.00942.x
Bakshi P, Bhat DJ, Wali VK, Sharma A, Iqbal M (2014) Growth, yield and quality of strawberry (Fragaria x ananassa Duch.) cv. Chandler as influenced by various mulching materials. Afr J Agri Res 9(7):701–706. https://doi.org/10.5897/AJAR2013.7983
Bajwa AA, Mahajan G, Chauhan BS (2015) Nonconventional weed management strategies for modern agriculture. Weed Sci 63(4):723–747. https://doi.org/10.1614/WS-D-15-00064.1
Bi J, Blanco JA, Seely B, Kimmins JP, Ding Y, Welham C (2007) Yield decline in Chinese-fir plantations: a simulation investigation with implications for model complexity. Can J For Res 37(9):1615–1630
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Cavaletti L, Monciardini P, Bamonte R, Schumann P, Rohde M, Sosio M, Donadio S (2006) New lineage of filamentous, spore-forming, gram-positive bacteria from soil. App Environ Microb 72:4360–4369
Cederlund H, Wessén E, Enwall K, Jones CM, Juhanson J, Pell M, Hallin S (2014) Soil carbon quality and nitrogen fertilization structure bacterial communities with predictable responses of major bacterial phyla. Appl Soil Ecol 84:62–68
Chalker-Scott L (2007) Impact of mulches on landscape plants and the environment—A review. J Environ Hortic 25(4):239–249. https://doi.org/10.24266/0738-2898-25.4.239
Chen FS, Niklas KJ, Liu Y, Fang XM, Wan SZ, Wang HM (2015) Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol 35:1106–1117
Chen L, Zhang J, Zhao B, Zhou G, Ruan L (2015) Bacterial community structure in maize stubble-amended soils with different moisture levels estimated by bar-coded pyrosequencing. Appl Soil Ecol 86:62–70
Chen W, Chen R, Zhang Y, Li J, Tigabu M, Ma X, Li M (2020) Cloning, characterization and expression analysis of the phosphate starvation response gene, ClPHR1, from Chinese Fir. Forests 11(1):104. https://doi.org/10.3390/f11010104
Cierjacks A, Pommeranz M, Schulz K, Almeida-Cortez J (2016) Is crop yield related to weed species diversity and biomass in coconut and banana fields of northeastern Brazil? Agric Ecosyst Environ 220:175–183
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503
Dai Z, Su W, Chen H, Barberán A, Zhao H, Yu M, Xu J (2018) Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro–ecosystems across the globe. Global Change Biol 24(5):3452–3461
DeBruyn JM, Nixon LT, Fawaz MN, Johnson AM, Radosevich M (2011) Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl Environ Microbiol 77:6295–6300
dos Santos TA, de Resende AS, da Silva FF, Machado AFL, Chaer GM (2019) Weed interference factor that affect the growth on an Atlantic Forest tree species. Embrapa Agrobiologia-Artigo em periódico indexado (ALICE). https://doi.org/10.14393/BJ-v35n2a2019-41820
Granot D, David-Schwartz R, Kelly G (2013) Hexose kinases and their role in sugarsensing and plant development. Front. Plant Sci 4:44. https://doi.org/10.3389/fpls.2013.00044
Guo YY, Yu HY, Yang MM, Kong DS, Zhang YJ (2018) Effect of drought stress on lipid peroxidation, osmotic adjustment and antioxidant enzyme activity of leaves and roots of Lycium ruthenicum Murr. seedling. Russ J Plant Physiol 65(2):244–250. https://doi.org/10.1134/S1021443718020127
Hamberg L, Malmivaara-Lämsä M, Löfström I, Vartiamäki H, Valkonen S, Hantula J (2011) Sprouting of Populus tremula L. in spruce regeneration areas following alternative treatments. Eur J For Res 130(1):99–106
Harper GJ, Comeau PG, Biring BS (2005) A comparison of herbicide and mulch mat treatments for reducing grass, herb, and shrub competition in the BC interior Douglas-fir zone—ten-year results. West J Appl For 20(3):167–176. https://doi.org/10.1093/wjaf/20.3.167
Hill E (2018) Status of herbicide-resistant weeds in Michigan. Michigan State University Extension Article. Online: https://www.canr.msu.edu/news/2018_status_of_herbicide_resistant_weeds_in_michigan
Hirose T, Scofield GN, Terao T (2008) An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci 174(5):534–543
Huang J, Hu B, Qi K, Chen W, Pang X, Bao W, Tian G (2016) Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur J Soil Biol 72: 35–41. https://doi.org/10.1016/j.ejsobi.2015.12.007
Weand MP, Arthur MA, Lovett GM, McCulley RL, Weathers KC (2010) Effects of tree species and N additions on forest floor microbial communities and extracellular enzyme activities. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2010.08.012
Kinney CA, Mandernack KW, Mosier AR (2005) Laboratory investigations into the effects of the pesticides mancozeb, chlorothalonil, and prosulfuron on nitrous oxide and nitric oxide production in fertilized soil. Soil Biol Biochemi 37(5):837–850
Kirby R (2006) Actinomycetes and lignin degradation. Adv Appl Microbiol 58:125–168
Knowe SA, Stein WI (1995) Predicting the effects of site preparation and protection on development of young Douglas-fir plantations. Can J Forest Res 25(9):1538–1547
Kuramae E, Gamper H, van Veen J, Kowalchuk G (2011) Soil and plant factors driving the community of soil-borne microorganisms across chronosequences of secondary succession of chalk grasslands with a neutral pH. FEMS Microbiol Ecol 77:285–294. https://doi.org/10.1111/j.1574-6941.2011.01110.x
Lee SM, Kong HG, Song GC, Ryu CM (2021) Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J 15(1):330–347
Li G, Niu W, Sun J, Zhang W, Zhang E, Wang J (2021) Soil moisture and nitrogen content influence wheat yield through their effects on the root system and soil bacterial diversity under drip irrigation. Land Degrad Dev. https://doi.org/10.1002/ldr.3967
Li Q, Liu C, Wang X, Jin Z, Song A, Liang Y, Müller WE (2018) Influence of altered microbes on soil organic carbon availability in karst agricultural soils contaminated by Pb-Zn tailings. Front Microbiol 9:2062. https://doi.org/10.3389/fmicb.2018.02062
Liu M, Liu J, Chen X, Jiang C, Wu M, Li Z (2018) Shifts in bacterial and fungal diversity in a paddy soil faced with phosphorus surplus. Biol Fert Soils 54(2):259–267
Liu X, Wang Y, Liu Y, Chen H, Hu Y (2020a) Response of bacterial and fungal soil communities to Chinese Fir (Cunninghamia lanceolate) long-term monoculture plantations. Front Microbiol 11:181
Liu Y, Fan X, Zhang T, He W, Song F (2020b) Effects of the long-term application of atrazine on soil enzyme activity and bacterial community structure in farmlands in China. Environ Pollut 262:114264. https://doi.org/10.1016/j.envpol.2020.114264
Löfgren S, Zetterberg T (2011) Decreased DOC concentrations in soil water in forested areas in southern Sweden during 1987–2008. Sci Total Environ 409(10):1916–1926
Lopes AR, Manaia CM, Nunes OC (2014) Bacterial community variations in an alfalfa-rice rotation system revealed by 16S rRNA gene 454-pyrosequencing. FEMS Microbiol Eco 87(3):650–663. https://doi.org/10.1111/1574-6941.12253
Kemmitt SJ, Wright D, Goulding KWT, Jones DL (2006) pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biol Biochem 38(5):898–911
Melgarejo P, Calín-Sánchez Á, Hernández F, Szumny A, Martínez JJ, Legua P, Martínez R, Carbonell-Barrachina ÁA (2012) Chemical, functional and quality properties of Japanese plum (Prunus salicina Lindl.) as affected by mulching. Sci Hortic 134:114–120. https://doi.org/10.1016/j.scienta.2011.11.014
Ma X, Heal KV, Liu A, Jarvis PG (2007) Nutrient cycling and distribution in different-aged plantations of Chinese fir in southern China. For Ecol Manag 243(1):61–74
Madhaiyan M, Poonguzhali S, Lee JS, Senthilkumar M, Lee KC, Sundaram S (2010) Mucilaginibacter gossypii sp. nov. and Mucilaginibacter gossypiicola sp. nov., plant-growth-promoting bacteria isolated from cotton rhizosphere soils. Int J Syst Evol Micro 60:2451–2457
Mahía J, Cabaneiro A, Carballas T, Díaz-Raviña M (2008) Microbial biomass and C mineralization in agricultural soils as affected by atrazine addition. Biol Fertil Soils 45(1):99–105. https://doi.org/10.1007/s00374-008-0318-y
Morris SJ, Blackwood CB (2015) The ecology of the soil biota and their function. In: Eldor EA (ed) Soil Microbiology, Ecology and Biochemistry, 4th edn, chap. 10. Academic, Cambridge, 273–309. https://doi.org/10.1016/B978-0-12-415955-6.00010-4
Newton M (2006) Taking charge in forest vegetation management. Can J Forest Res 36(10):2357–2363
Nie Y, Wang M, Zhang W, Ni Z, Hashidoko Y, Shen W (2018) Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci Total Environ 624:407–415
Niu J, Rang Z, Zhang C, Chen W, Tian F, Yin H, Dai L (2016) The succession pattern of soil microbial communities and its relationship with tobacco bacterial wilt. BMC Microbiol 16(1):1–10. https://doi.org/10.1186/s12866-016-0845-x
Pankratov TA, Tindall BJ, Liesack W, Dedysh SN (2007) Mucilaginibacter paludis gen. nov., sp. nov. and Mucilaginibacter gracilis sp. nov., pectin-, xylan-and laminarin-degrading members of the family Sphingobacteriaceae from acidic Sphagnum peat bog. Int J Syst Evol Micro 57:2349–2354
Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP (2015) In vitro and in vivo plant growth promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associates with medicinal plants. PLoS One 10:e0139468
Peachey RE, Landgren CG, Miller TW (2017) Weed and vegetation management strategies in Christmas trees. Oregon State University
Purcell AH, Hopkins DL (1996) Fastidious xylem-limited bacterial plant pathogens. Ann Rev P hytopathol 34(1):131–151
Rawat SR, Männistö MK, Bromberg Y, Häggblom MM (2012) Comparative genomic and physiological analysis provides insights into the role of Acidobacteria in organic carbon utilization in Arctic tundra soils. FEMS Microbiol Ecol 82:341–355. https://doi.org/10.1111/j.1574-6941.2012.01381.x
Saha D, Cregg BM, Sidhu MK (2020) A review of non-chemical weed control practices in Christmas tree production. Forests 11(5):554. https://doi.org/10.3390/f11050554
Sannino F, Gianfreda L (2001) Pesticide influence on soil enzymatic activities. Chemosphere 45:417–425
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Weber CF (2009) Introducing mothur: open-source, platformindependent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schneider WG, Knowe SA, Harrington TB (1998) Predicting survival of planted Douglas-fir and ponderosa pine seedlings on dry, low-elevation sites in southwestern Oregon. New For 15(2):139–159. https://doi.org/10.1023/A:1006523404870
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. https://doi.org/10.1186/gb-2011-12-6-r60
Sun K, Sun HG, Qiu ZH, Liu Q (2021) Comparative analyses of Phyllosphere bacterial communities and metabolomes in newly developed needles of Cunninghamia lanceolata (Lamb.) Hook. at Four Stages of Stand Growth. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.717643
Tan Y, Cui Y, Li H, Kuang A, Li X, Wei Y, Ji X (2017) Diversity and composition of rhizospheric soil and root endogenous bacteria in Panax notoginseng during continuous cropping practices. J Basic Microb 57:337–344
Tian D, Xiang W, Chen X, Yan W, Fang X, Kang W, Peng Y (2011) A long-term evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. Forestry 84(4):411–418
Tian J, He N, Hale L, Niu S, Yu G, Liu Y, Zhou J (2018) Soil organic matter availability and climate drive latitudinal patterns in bacterial diversity from tropical to cold temperate forests. Funct Ecol 32(1):61–70
Ullah A, Akbar A, Luo Q, Khan AH, Manghwar H, Shaban M, Yang X (2019) Microbiome diversity in cotton rhizosphere under normal and drought conditions. FEMS Microbiol Ecol 77(2):429–439. https://doi.org/10.1007/s00248-018-1260-7
Umadevi P, Anandaraj M, Srivastav V, Benjamin S (2017) Trichoderma harzianum MTCC 5179 impacts the population and functional dynamics of microbial community in the rhizosphere of black pepper (Piper nigrum L). Braz J Microbiol 49:463–470. https://doi.org/10.1016/j.bjm.2017.05.011
Vogel JG, Suau LJ, Martin TA, Jokela EJ (2011) Long-term effects of weed control and fertilization on the carbon and nitrogen pools of a slash and loblolly pine forest in north-central Florida. Can J For Res 41(3):552–567
Wang C, Zheng M, Song W, Wen S, Wang B, Zhu C, Shen R (2017) Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol Biochem 113:240–249
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb 73:5261–5267
Wang Q, Wang C, Yu WW, Turak A, Chen DW, Huang Y, Ao J, Jiang Y, Huang Z (2018) Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese fir plantations. Front Microbiol 9:1543
Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Kuske CR (2009) Three genomes from the phylum Acidobacteria provide insights into the lifestyles of these microorganism in soils. Appl Environ Microbiol 75:2046–2056
Watkins KL, Veum TL, Krause GF (1987) Total nitrogen determination of various sample types: A comparison of the Hach, Kjeltec, and Kjeldahl methods. J Assoc Off Anal Chem 70:410–412. https://doi.org/10.1093/jaoac/70.3.410
Zhang Z, Zhang Q, Cui H, Li Y, Xu N, Lu T, Chen J, Penuelas J, Hu B, Qian H (2022) Composition identification and functional verification of bacterial community in disease-suppressive soils by machine learning. Environ Microbiol 00(00):00–00
Zhou J, Jiang X, Wei D, Zhao B, Ma M, Chen S, Li J (2017) Consistent effects of nitrogen fertilization on soil bacterial communities in black soils for two crop seasons in China. Sci Rep 7(1):1–10. https://doi.org/10.1038/s41598-017-03539-6
Zimdahl RL (2007) Fundamentals of weed science. Academic, Elsevier, Location
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (grant no. 2021YFD2201303-03). We thank Xiangying Jiang and Wenliang Zhao for their help for our project. We thank Robert McKenzie, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Iain Paul Hartley.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 128 KB)
Rights and permissions
About this article
Cite this article
Wang, S., Sun, H., Santos, E. et al. Soil microbial communities, soil nutrition, and seedling growth of a Chinese fir (Cunninghamia lanceolata (Lamb.) Hook.) plantation in response to three weed control methods. Plant Soil 480, 245–264 (2022). https://doi.org/10.1007/s11104-022-05578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05578-z