Abstract
Aims
Hydraulic redistribution (HR) enhances water resources for neighboring crops in silvopastoral agroforestry (AF). Here, we tested whether and to what extent water stressed shallow-rooted neighboring plants benefit from water redistributed by deep-rooted poplar plants.
Methods
We conducted trace experiments with deuterated water (2H2O) in greenhouse soil column experiments. We measured hydraulic lift (HL) by poplars grown at two levels of soil drying and estimated the amount of hydraulically lifted water. In a parallel experiment we grew poplars and barley (Hordeum vulgare) in two columns connected via a small cross-rooting segment.
Results
Soil moisture measurements and stable isotope signatures of soil and xylem water proved the occurrence of HL in poplar. Additionally, stable isotopes proved the transport of water from deep roots of poplars to shallow roots of barley.
Conclusions
In conclusion, the experiments showed that poplars are capable to redistribute water during drought spells and that this water can facilitate plant growth of shallow-rooted crops. This result implies evidence for an enhanced soil water supply of plants in agroforest systems under drought conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is one of the most limiting resources for plant growth, survival and productivity. Agricultural intensification at the expense of biodiversity and productivity of natural ecosystems, in addition to increasing mean temperatures and declining annual precipitation aggravate water scarcity during summer drought spells. Drought risks put intense pressure on water use efficiency in agricultural managed soils and landscapes. Therefore, soil water resources should be well managed and avoidable water losses reduced.
Silvopastoral AF combines silviculture with conventional agriculture by implementing tree strips into crop land and it offers diverse opportunities to optimize water use efficiency, especially during dry summer periods. Moreover, deep rooting trees can increase plant available water by relocating soil water from deep, moist soil layers to dry, shallow layers via the root system. HR describes the movement of water from wet to dry soil regions via the roots along water potential gradients in the soil in periods of low transpiration rates (Burgess et al. 1998, 2000; Prieto et al. 2012; Richards and Caldwell 1987). During high transpiration rates, leaf water potential is significantly lower compared to soil water potential in the rhizosphere and water moves from plant roots to foliage. HR takes place when reversed potential gradients occur – i.e. when soil water potential is lower than the water potential. HR is passive in the sense that its driving force is a gradient in water potential and does not require active pumping by the root system. However, plants could regulate HR by modifying the permeability of roots and that of the rhizosphere, for instance by secreting mucilage or losing contact with the soil (Carminati et al. 2010, 2013). A recent study by Yan et al. (2020) shows that HR can be actively regulated by root hairs and rhizodeposition. Depending on soil characteristics, root distribution and soil water distribution, HR can occur upwards (i.e. hydraulic lift (HL)) (Caldwell and Richards 1989), downwards (Burgess et al. 1998; Schulze et al. 1998; Smith et al. 1999) and horizontal (Brooks et al. 2002).
The impacts of ecological and hydrological conditions on the intensity of HR vary among ecosystems, soil textures and plant communities (Neumann and Cardon 2012). It is suggested that the intensity of HR decreases as a function of the amount of sand in the soil (Wang et al. 2009). HR requires large gradients in water potential, as water is released from the roots along this gradient. In sandy soils the root-soil contact fraction decreases as the soil dries and the resistance to water movement is greatly increased (Passioura 1991), implying that less water can be released into dryer soil (Vetterlein and Marschner 1993). In this study different moisture gradients were used to identify HR in sandy soils.
HR could be beneficial in AF systems as it can supply water to shallow-rooted crops growing next to trees (Liste and White 2008; Ludwig et al. 2004; Moreira et al. 2003).This was shown in greenhouse experiments (Hawkins et al. 2009). Additionally, research in the semi-arid Sahel has shown that two native shrubs not only perform hydraulic lift (Kizito et al. 2006, 2007, 2012) but can also assist millet through in-season drought (Bogie et al. 2018). Moreover, redistributed water extends the life span of roots and preserves the root-soil contact in drying soils and at the same time reduces the occurrence of root embolism under water stress (Bauerle et al. 2008; Domec et al. 2004).
A method to experimentally investigate HR is the use of water stable isotopes. The application of 2H2O water as an environmental tracer offers many advantages due to the fact that it is part of the water molecule, it is a non-radioactive and eco-friendly substance, without any damaging characteristics for the application in natural ecosystems, and it is considered to be conservative (Koeniger et al. 2010). Because root water uptake and transport through the xylem in general does not change the isotopic composition of the soil water (Zimmermann et al. 1967), 2H2O was successfully used to identify rooting depth of plants in subtropical (Kulmatiski et al. 2010) and semiarid environments (Beyer et al. 2016), as well as the occurrence of a benefit of HR for drought tolerance (Dawson 1993; Hawkins et al. 2009). Furthermore, Bogie et al. 2018 used labeled water (²H2O ) to show that interplanted shrubs “bioirrigate” hydraulicly lifted water to adjacent millet plants. Although it is accepted that HR is important for drought tolerance, less is known about tree-crop interactions in temperate agricultural systems which are well watered during autumn, winter and beginning of spring, while they might face drought spells during the summer.
The objective of this study was to test the hypothesis that water stressed crops benefit from hydraulic redistributed water from deep rooting trees. In particular, we tested to what extent different soil moisture gradients have an impact on the amount of HR and if poplar trees redistribute water to neighboring shallow rooted barley plants.
Material and method
Experimental set up and plant culture
The experiments were performed in a greenhouse with no additional light source or automatic shading. Plants were exposed to natural diurnal light and temperature variations. The windows were kept open to allow air exchange and level out the relative humidity.
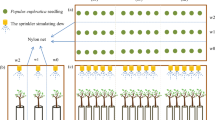
The first experimental setup had a completely randomized block design with four replications that had the following treatments: (A) upmost soil dry, middle and subsoil at field capacity; and (B) upmost and middle soil layers dry and subsoil at field capacity. Eight identically constructed Plexiglas® columns A&B (height 100 cm, diameter 30 cm) were filled with fine quartz sand (0,1 − 0,2mm, Fig. 1). In each column, three sand layers were hydraulically separated by two fine gravel (2.0–5.0mm) layers acting as capillary boundary and preventing water redistribution across the layers. Perforated metal tubes (diameter 0.5 cm, length 28 cm) allowed for irrigation of each soil layer separately and remained in the column for the whole times of the experiment. The top soil layer was irrigated from the soil surface.
The second experimental setup had two replicates that had the following treatment: C) topmost soil layer dry and subsoil around field capacity, with the adjacent column dry. Two soil columns were connected via a cross-rooting section (Fig. 1: C and Cbarley). A Plexiglas® tube (10 cm length and 10 cm in diameter) connected the two soil columns at a height of 50 cm. In contrast to the first setup, the experiment C had two hydrologically divided soil layers, whereas the soil column Cbarley was filled without any layer with sand (same material was used as in first experimental setup).
Poplar (Populus nigra x Populus maximowiczii) cuttings were grown in water until roots sprouted and then they were planted into soil columns. They were grown for seven months to ensure a vigorous root growth into all soil layers. Barley (Hordeum vulgare) was sown at the soil surface in soil column Cbarley 28 days before the tracer experiment started. During the first five weeks of the experiment the poplar cuttings were irrigated and fertilized (N + P2O5 + K2O+(MgO + S)) once a week with 60ml of fertilized water, to maintain the soil above field capacity. Afterwards, irrigation was extended to the middle layers A2 and B2. After seven weeks the lowest layers A3, B3 and C2 were irrigated. During the first months the amount of irrigation was adapted to observed plant health and rooting depth and was increased over the time of the whole experiment. After three months A1, B1 and C1 were fertilized again. Similarly, Cbarley was irrigated to attract the poplar roots to this soil column. Right after sowing the barley, Cbarley was irrigated and fertilized again (details are found in the supplemental material).
Establishing soil moisture gradients
In one soil column of each experimental set-up the volumetric soil moisture content was measured with dielectric SM 300 sensors (Delta-T Devices Ltd, Cambridge, United Kingdom). SM 300 sensors were placed in the middle of each soil layer, except for Cbarley, where the sensors were placed at 10 cm soil depth. SM300 measured soil moisture content at a time interval of 2 min for the whole time of plant culture and tracer experiment. Irrigation was adapted 10 weeks prior to tracer injection to allow the trees and roots to adapt to established soil moisture gradients.
Experiments A and B served to mimic two different soil water content distributions and to assess their impact on HR. Soil columns A and B were irrigated equally until specific soil moisture gradients were established. Replicates of A were mainly irrigated in the middle (A2) and lowest (A3) soil layers, whereas replicates of B were irrigated mainly in the lowest (B3) soil layers. Due to root water uptake and evaporation, soil moisture contents close to the permanent wilting point (PWP) were reached in the upmost layers (A1, B1) and middle soil layer (B2) (Fig. 2). The lowest layers (A3, B3) were maintained at or above field capacity. We measured the water retention curve of used sandy soil by using a pressure chamber apparatus. The water content at a matric potential of -1.5 MPa (pF 4.2) is referred to as the wilting point, and it was equal to 0.04 volumetric water content.
In experiment C the samples were first irrigated and fertilized in both columns with the intention to attract the poplar roots to grow across the cross-rooting section. During the first two months, poplars showed slower growing rates compared to those in the first experiment, therefore irrigation and fertilization was intensified in soil column C. With the start of regulating the soil moisture gradients, irrigation was then again enhanced in column Cbarley, to foster poplar root growth into Cbarley and on the other side to ensure successful sowing of barley.
Sampling and water extraction
Two hours before tracer injection, soil and plant samples were collected to determine the background values of 2H in soil water and xylem sap. The tracer was injected at 10 a.m. Soil samples were taken in the upper soil layer at 5 cm soil depth. For xylem samples small segments of the branch were cut off, the bark and phloem was removed rapidly and immediately after sampling the soil and xylem were stored airtight in weighted vials for the cryogenic vacuum extraction and thereafter frozen at -18°C. To ensure a sufficient amount of extractable water at least 10 g of soil and 5 g of plant material were sampled.
The tracer injection was conducted simultaneously in all samples to allow comparability between replicates. 1000 ml of deuterated water (10 atom% 2H) were injected via the irrigation tubes in the lowest soil layers (A3, B3, C2). Subsequently, soil and plant samples were collected at 10, 24, 34, 48, 58, 72 hours after injection of the tracer. Soil samples were taken in A1, B1 and C1 at 2.5 cm depth. Xylem was sampled at the same time as soil samples and additionally at 3, 5, and 8 hours after injection.
Soil and xylem water was extracted with cryogenic vacuum distillation (West et al. 2006). Xylem samples were extracted for 60 min and soils for 45 min with a heating source of approx. 100°C. To determine and secure the completeness of water extraction, samples were weighed before and after extraction and again after 24 h oven drying at 105°C.
Stable isotope analyses were done at the Centre for Stable Isotope Research and Analysis (KOSI) at the University of Göttingen. Xylem and soil waters were analyzed with continuous flow EA/TC isotope ratio mass spectroscopy (Delta V™ plus IRMS, Thermo Fischer Scientific™, Bremen, Germany).
Root distribution
After the tracer experiments, the soil columns were opened and roots were sampled in each soil layer. Living roots were assorted for fine (< 2 mm) and coarse (> 2 mm) roots, by cutting the roots into pieces and sieved them. Afterwards they were oven dried for 48 h at 105°C (until constant weight was achieved) and weighted to calculate the specific dry root distribution in each soil layer (referring to DIN ISO 11,465).
Data analysis
To clearly state whether the injected tracer was abundant in the samples, we used the criteria first proposed by Kulmatiski et al. (2010) and later adapted by Beyer et al. (2016). If the detected stable isotope signatures in extracted soil or xylem water were higher than four times the standard deviation of the background sample, we assumed the tracer to be present in the sample.
Continuously measured water contents were smoothed with Savitzky-Golay convolution to improve the signal-noise ratio of the two minutes measurements with a 60 min moving window averaging (Savitzky and Golay 1964).
Calculations
Volume of hydraulic redistributed water in the upper layer
We calculated the HL in volume of water per time (here per day) (Eq. 1) with the help of the mass balance of 2H2O, under the assumption that that there is no significant change in water content in the upper soil layer (i.e. we assume that the water flowing out from the root at night is taken up during the next day). In addition we assume that the concentration of 2H2O flowing out of the roots is equal to the measure concentration in the poplar xylem Cx. From these assumptions we derive the following equation:
with Cx [atom%] being the 2H2O concentration in the poplar xylem and Cs [atom%] being the 2H2O concentration in the upper layer (after one day), HL [cm3 d− 1] describing the hydraulic lift in volume of water per time, θ [-] being the soil water content in the upper layer, Volupper layer [cm3] being the volume of the upper soil layer and dCs/dt [atom% d− 1] describing the change in the 2H2O concentration in the upper soil layer over one day.
Results
Root distribution
In experiments A and B the density of coarse roots was the highest in the upmost soil layers (Table 1). The amount of coarse poplar roots decreased by 80 % in A and ca. 75 % in B with increasing soil depth. On the contrary, fine roots did not show this typical root pattern, with more than half of the total fine root mass being found in the lowest soil layers A3 and B3. The mass of fine roots was highly dependent on soil water content. High amounts of fine root density were associated with high soil water contents, whereas soil layer with lower water content and therefore less plant available water had lower fine root densities (Table 1). This becomes particularly obvious comparing A2 and B2. While in A2 soil moistures were close to field capacity, soil water contents in B2 were closer to the PWP and root density decreased by more than 50 %.
In comparison to A and B, root mass in columns C and Cbarley was lower, especially regarding the total amount of fine roots. Fine root density was constant over depth, whereas coarse root density decreased by almost 90 %. Poplar fine roots were observed in Cbarley. However, no barley roots were found in soil column C, indicating that poplar did use the crossing-section to root in Cbarley.
Soil water content
Soil water contents highly differed between the treatments. A steep increase in water content followed irrigation, and then water content decreased due to drainage, evaporation and root water uptake. In experiment (A), soil moisture increased with soil depth from close to PWP in A1 to more than field capacity in the lowest soil layer A3 (Table 1; Fig. 2). B1 and B2 were close to PWP while B3 remained wet. The soil columns in experiment C were overall drier compared to A and B. Soil moisture in C2 was close to field capacity, whereas C1 and Cbarley were close to PWP. Small diurnal changes in soil water contents were visible in all columns and all depths, with small amplitudes of moisture gain during the night and water loss over the day. However, these oscillations are within the precision of the sensors.
Stable isotope data
Experimental setup 1
Xylem 2H values increased under both drying stages (A and B) to values above artificial tracer threshold within the first 5 hours after tracer injection (Fig. 3), showing clear artificial tracer abundance. Stable isotope values of trees in experiment A ranged between 0.5 and 0.8 atom% (mean 2H over time 0.68 atom% ± 0.10), whereas trees grown under drier conditions (B) showed values ranging from 1.5 to 1.9 atom% (mean 2H over time 1.52 atom% ± 0.30). 2H values of poplar A indicated mixing of waters from the two wet layers, while poplar confronted with severer soil drying mainly takes up water in B3. Assuming homogenous mixing of injected and present water, we obtain the poplar B takes up water almost entirely in B3.
Tracer recovery in poplar xylem and upper soil under two different soil moisture gradients representing varying drought stress. Values are means of replicates ± standard deviation and significance to background value (± 4 * standard deviation) is marked. Clearly artificial 2H signal is marked above where tracer is abundant
Clearly artificial 2H values in soil water are found 48 h after injection in the topmost soil layers of A1 (0.16 atom% ± 0.06) and already after 34 h in B1 (0.33 atom% ± 0.15). The lower soil water stable isotopes values of A1 can be explained by the mixing and multilayered root water uptake of poplar A, resulting in lower 2H values of redistributed water compared to the set up B. Towards the end of the experiment, 2H values decreased in experiment A.
Experimental setup 2
2H values in poplar xylem slightly increased during the first three hours after injection, with a decline in the evening of day one (Fig. 4). During the second day 2H values rose up to 0.37 atom% (± 0.028) and again declined during the second night. The same pattern with a time shift of 12 h was observed in the 2H values of the barley xylem. Whereas a first peak was seen in the evening of the first day, values during the second day stayed rather low and increased again remarkably during the second night up to 0.026 atom% (± 0.0002), followed by a repetitive decrease over day three.
Soil water isotopes did not change much during the days and no clear artificial tracer abundance can be seen, although 2H signatures rose up to 0.055 atom% ± 0.023 at day three.
Amount of hydraulically lifted water
Based on the mass balance for 2H2O we were able calculating the HL in volume of water per day (Eq. 1). Therefore we used the daily mean water content and the isotopic values after 24 hours after the tracer injection. For the replicates of A we have a flux of 67,45 cm3 d− 1 and for replicates of B a flux of 66,25 cm3 d− 1.
Discussion
Our results support the hypothesis that water stressed shallow rooted crops can greatly benefit from hydraulic redistributed water by neighboring deep rooted trees. The main factor driving the occurrence of HR is a steep soil water potential gradient between topsoil and subsoil.
Diurnal changes in water content are an indication of HR (Caldwell and Richards 1989). However, parts of the fluctuations of soil water content are caused by water redistribution along the soil profile. Therefore, from small amplitudes of changes in soil moisture content only it is not possible to state whether hydraulic redistribution takes place. To distinguish between these simultaneously occurring processes, we used 2H as naturally abundant stable water isotope tracer. The applied high 2H concentrations (> 0.02 atom%) in the hydrological separated subsoil allow to prove the occurrence of HR.
Stable isotope values are affected by the mixing of tracer and present soil water after injection. Although diffusion and convection due to gravitational forces and density variations take place over the whole time, homogenous mixture and equilibration of 2H might not be achieved during the observation time. This and the effect that poplars did not have identical root systems, although with no clear differences in root mass within one experimental set up (Table 1), result in high standard deviations (Fig. 3). Nevertheless, due to identical fertilization and watering, we assume that transpiration and root water uptake rates were not significantly different along the replicates.
The amount of hydraulically lifted water was similar in both drying scenario, A and B. It means that water in the lowest soil layers was sufficient to sustain HL. In this case the available water did not directly influence the effect of HR, as long as enough water was available to properly meet the demand of transpiration and growth (Liste and White 2008).
The 2H values in the poplar xylem show fluctuation during day and night: enriched in the evening and depleted (in relation to the day before) in the morning. Enrichment of 2H was caused by root water uptake in the subsoil driven by the transpiration demand. During night time diffusion of water between xylem, phloem and cortex might explain the low 2H values in the next morning (poplar A day 2&4, poplar B day 2&3 and poplar C day 3&4). Over the second night the soil water stable isotope values increased due to HL (A). The next morning the redistributed water was taken up by poplars in addition to subsoil root water uptake. This process outweighed the diffusive interchange of waters in the plant during the night and will causing an enrichment of tracer over the following day.
It has to be noted that hydraulically redistributed water in the soil always represents a mixture of water pools: subsoil water taken up during the day, previously redistributed soil water and water present in the plant itself. Once water is relocated in the upper soil layers, evaporative fractioning will occur, influencing the soil water isotopy and causing a decreasing natural depletion of 1H with increasing soil depth (Allison et al. 1983). In our experiments, the impact of natural fractioning in comparison with the shown enriched values of 2H was negligible.
As a consequence of root water uptake of hydraulically redistributed water, 2H2O values in barley increased strongly by the next morning (third day). We assume that hydraulically redistributed water was much easier accessible compared to residual water in the upper soil. Therefore, barley plants will use redistributed water first and by the end of the day the ratio of origin water pools will shift towards residual soil water explaining the decreasing 2H over the day. Our results of the reuse of redistributed water by neighboring plants and seedlings agree with findings among other studies Dawson 1993; Bogie et al. 2018; Hawkins et al. 2009; Liste and White 2008; Ludwig et al. 2004; Moreira et al. 2003; Muler et al. 2018).
Our calculations indicate high amounts of water redistributed by poplar trees. Considered the young age and growing conditions (quartz sand, limited space of rooting depth, low nutrient availability, very low Corg and defined irrigation sequences) of the poplars grown in the greenhouse, one might expect even greater HL with older trees and larger root system. On the other side, the soil conditions in our experimental set up were extreme because of the high differences in water availability between dry and wet zones in a short distance. Therefore, the amount and impact of HL under natural field conditions needs further studies.
Further studies should also investigate in more details the mechanisms and plants traits favoring HL. For instance, it should be clarified to what extent HL is affected by root traits such as root diameter, root hairs, root hydraulic permeability, as well as the presence of mycorrhiza, which can enhance HL due to their extension in soil and enlarged active surface area.
According the “stress-gradient hypothesis” postulated by Bertness and Callaway (1994), beneficial plant interactions have increasingly positive effects when the environmental stresses increase, whereas competitive interactions become more intense under less severe stresses. Michalet et al. (2014) added that at extremely harsh environmental conditions beneficial interactions collapse and competition between plant organisms dominate. Although it has been shown that the facilitative effects of HR might be less relevant than the competition for water in semi-arid regions (Ludwig et al. 2004), in temperate regions with moderate stress HR can overcome the competition for water. We could show that HR mainly enhances water resources for the deep rooting tree itself, by increasing the amount of plant available water to facilitate next days’ water need. In addition, we have found that redistributed water was taken up by the neighboring barley, facilitating their growth.
Applying these results to AF systems is not straightforward, as both temporal and special scales of our experiment were limited to greenhouse conditions. However, combination of isotopic sampling, transpiration measurements and soil moisture measurements over the relevant depths could be performed in the field and provide estimates of HR in AF. Such data could support the implementation of trees into crop land and their potential to enhance soil water resources and maintain shallow rooted crop growth, preventing high harvest losses due to prolonged summer drought spells. The limiting factor for HR as natural enhancement for water resources is the overall availability of water in the subsoil. We could show in this experiment that as long as sufficient water resources are available in the subsoil, HR by trees could sustain crop growth in an AF system.
References
Allison GB, Barnes CJ, Hughes MW (1983) The distribution of deuterium and 18O in dry soils 2. experimental. J Hydrol 64(1–4):377–397. https://doi.org/10.1016/0022-1694(83)90078-1
Bauerle TL, Richards JH, Smart DR, Eissenstat DM (2008) Importance of internal hydraulic redistribution for prolonging the lifespan of roots in dry soil. Plant Cell Environ 31(November):177–186. https://doi.org/10.1111/j.1365-3040.2007.01749.x
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends Ecol Evol 9(5):191–193. https://doi.org/10.1016/0169-5347(94)90088-4
Beyer M, Koeniger P, Gaj M, Hamutoko JT, Wanke H, Himmelsbach T (2016) A Deuterium-based labeling technique for the investigation of rooting depths, water uptake dynamics and unsaturated zone water transport in semiarid environments. J Hydrol 533(February):627–643. https://doi.org/10.1016/j.jhydrol.2015.12.037
Bogie NA, Bayala R, Diedhiou I, Conklin MH, Fogel ML, Dick RP, Ghezzehei TA (2018) Hydraulic redistribution by native sahelian shrubs: bioirrigation to resist in-season drought. Front Environ Sci 6(September):98. https://doi.org/10.3389/fenvs.2018.00098
Brooks JR, Meinzer FC, Coulombe R, Gregg J (2002) Hydraulic redistribution of soil water during summer drought in two contrasting pacific northwest coniferous forests. Tree Physiol 22(15–16):1107–1117. https://doi.org/10.1093/treephys/22.15-16.1107
Burgess SSO, Mark A, Adams NC, Turner, Ong CK (1998) The redistribution of soil water by tree root systems. Oecologia 115(3):306–311. https://doi.org/10.1007/s004420050521
Burgess S, John S, Pate MA, Adams, Todd ED (2000) Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes. Ann Bot 85(2):215–224. https://doi.org/10.1006/anbo.1999.1019
Caldwell MM, Richards JH (1989) Hydraulic lift: water efflux from upper roots improves effectiveness of water uptake by deep roots. Oecologia 79(1):1–5. https://doi.org/10.1007/BF00378231
Carminati A, Moradi AB, Vetterlein D, Vontobel P, Lehmann E, Weller U, Vogel H-J, Oswald SE (2010) Dynamics of soil water content in the rhizosphere. Plant Soil 332(1–2):163–176. https://doi.org/10.1007/s11104-010-0283-8
Carminati A, Vetterlein D, Koebernick N, Blaser S, Weller U, Vogel H-J (2013) Do roots mind the gap? Plant Soil 367(1–2):651–661. https://doi.org/10.1007/s11104-012-1496-9
Dawson TE (1993) Hydraulic lift and water use by plants: implications for water balance, performance and plant-plant interactions. Oecologia 95(4):565–574. https://doi.org/10.1007/BF00317442
Domec J-C, Warren JM, Meinzer FC, Brooks JR, Coulombe R (2004) Native root xylem embolism and stomatal closure in stands of douglas-fir and ponderosa pine: mitigation by hydraulic redistribution. Oecologia 141(1):7–16. https://doi.org/10.1007/s00442-004-1621-4
Hawkins Heidi-J, Hettasch H, West AG (2009) Hydraulic redistribution by Protea ‘Sylvia’ (Proteaceae) facilitates soil water replenishment and water acquisition by an understorey grass and shrub. Funct Plant Biol 36(8):752. https://doi.org/10.1071/FP09046
Kizito F, Dragila M, Sène M, Lufafa A, Diedhiou I, Dick RP, Selker JS et al (2006) Seasonal soil water variation and root patterns between two semi-arid shrubs co-existing with pearl millet in Senegal, West Africa. J Arid Environ 67(3):436–455. https://doi.org/10.1016/j.jaridenv.2006.02.021
Kizito F, Sène M, Dragila MI, Lufafa A, Diedhiou I, Dossa E, Cuenca R, Selker J, Dick RP (2007) Soil water balance of annual crop–native shrub systems in senegal’s peanut basin: the missing link. Agric Water Manag 90(1–2):137–148. https://doi.org/10.1016/j.agwat.2007.02.015
Kizito F, Dragila MI, Senè M, Brooks JR, Meinzer FC, Diedhiou I, Diouf M et al (2012) Hydraulic redistribution by two semi-arid shrub species: implications for Sahelian agro-ecosystems. J Arid Environ 83(August):69–77. https://doi.org/10.1016/j.jaridenv.2012.03.010
Koeniger P, Leibundgut C, Link T (2010) Stable isotopes applied as water tracers in column and field studies. Org Geochem 41(1):31–40. https://doi.org/10.1016/j.orggeochem.2009.07.006
Kulmatiski A, Beard KH, Verweij RJT, February EC (2010) A depth-controlled tracer technique measures vertical, horizontal and temporal patterns of water use by trees and grasses in a subtropical savanna. New Phytol 188(1):199–209. https://doi.org/10.1111/j.1469-8137.2010.03338.x
Liste H-H, White JC (2008) Plant hydraulic lift of soil water – implications for crop production and land restoration. Plant Soil 313(1–2):1–17. https://doi.org/10.1007/s11104-008-9696-z
Ludwig F, Dawson TE, Prins HHT, Berendse F, Kroon H (2004) Below-ground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol Lett 7(8):623–631. https://doi.org/10.1111/j.1461-0248.2004.00615.x
Michalet R, Bagousse-Pinguet YL, Maalouf J-P, Lortie CJ (2014) Two alternatives to the stress-gradient hypothesis at the edge of life: the collapse of facilitation and the switch from facilitation to competition.” Edited by Michael Palmer. J Veg Sci 25(2):609–613. https://doi.org/10.1111/jvs.12123
Moreira MZ, Scholz FG, Bucci SJ, Sternberg LS, Goldstein G, Meinzer FC, Franco AC (2003) Hydraulic lift in a neotropical savanna. Funct Ecol 17(5):573–581. https://doi.org/10.1046/j.1365-2435.2003.00770.x
Muler AL, van Etten EJB, Stock WD, Howard K, Froend RH (2018) Can hydraulically redistributed water assist surrounding seedlings during summer drought? Oecologia 187(3):625–641. https://doi.org/10.1007/s00442-018-4158-7
Neumann RB, Cardon ZG (2012) The magnitude of hydraulic redistribution by plant roots: a review and synthesis of empirical and modeling studies: Tansley review. New Phytol 194(2):337–352. https://doi.org/10.1111/j.1469-8137.2012.04088.x
Passioura J (1991) Soil structure and plant growth. Aust J Soil Res 29(6):717. https://doi.org/10.1071/SR9910717
Prieto I, Armas C, Francisco I, Pugnaire (2012) Water release through plant roots: new insights into its consequences at the plant and ecosystem level: Tansley Review. New Phytol 193(4):830–841. https://doi.org/10.1111/j.1469-8137.2011.04039.x
Richards JH, Caldwell MM (1987) Hydraulic lift: substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 73(4):486–489. https://doi.org/10.1007/BF00379405
Savitzky A, Golay M (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36.8:1627–1639
Schulze E-D, Caldwell MM, Canadell J, Mooney HA, Jackson RB, Parson D, Scholes R, Sala OE, Trimborn P (1998) Downward flux of water through roots (i.e. inverse hydraulic lift) in dry Kalahari sands. Oecologia 115(4):460–462. https://doi.org/10.1007/s004420050541
Smith DM, Jackson NA, Roberts JM, Ong CK (1999) Reverse flow of sap in tree roots and downward siphoning of water by Grevillea robusta. Funct Ecol 13(2):256–264. https://doi.org/10.1046/j.1365-2435.1999.00315.x
Vetterlein D, Marschner H (1993) Use of a microtensiometer technique to study hydraulic lift in a sandy soil planted with pearl millet (Pennisetum americanum [L.] Leeke). Plant Soil 149(2):275–282. https://doi.org/10.1007/BF00016618
Wang X, Tang C, Guppy CN, Sale PWG (2009) The role of hydraulic lift and subsoil P placement in P uptake of cotton (Gossypium hirsutum L.). Plant Soil 325(1–2):263–275. https://doi.org/10.1007/s11104-009-9977-1
West AG, Shela J, Patrickson, Ehleringer JR (2006) Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Commun Mass Spectrom 20(8):1317–1321. https://doi.org/10.1002/rcm.2456
Yan J, Bogie NA, Ghezzehei TA (2020) Root uptake under mismatched distributions of water and nutrients in the root zone. Biogeosciences 17(24):6377–6392. https://doi.org/10.5194/bg-17-6377-2020
Zimmermann U, Münnich KO, Roether W (1967) Downward movement of soil moisture traced by means of hydrogen isotopes. Isot Tech Hydrol Cycle 11:28–36
Acknowledgements
This work was partly funded by the German Federal Ministry for Education and Research (BMBF) within the SIGNAL project (Sustainable Intensification of Agriculture Through Agroforestry). We like to thank Dirk Böttger for his technical support, as well as Wolf Hansel and Johannes Sauer for their work and effort during the whole greenhouse experiment. Last but not least, we kindly thank the editors and the reviewers for their ideas and constructive comments to improve this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Rafael S. Oliveira.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 252 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerjets, R., Richter, F., Jansen, M. et al. Hydraulic redistribution by hybrid poplars (Populus nigra x Populus maximowiczii) in a greenhouse soil column experiment. Plant Soil 463, 145–154 (2021). https://doi.org/10.1007/s11104-021-04894-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04894-0