Abstract
Background and aims
Vanadium (V), niobium (Nb), and tantalum (Ta), recognized as Technology-Critical Element (TCE), are highly growing in demand for industrial development. Despite their economic relevance, little is known about their environmental concentrations, especially in marine ecosystems like mangroves. This paper describes concentrations and distribution patterns of Group Va elements (V, Nb and Ta) in plant organs and sediments from diverse mangroves of the Indian Sundarbans.
Method
Sediment cores and plant organs of eight dominant mangrove species were sampled and analyzed for V, Nb and Ta by ICP-MS. Stable carbon isotope (δ13C) in mangrove leaves were analyzed by EA-IRMS.
Result
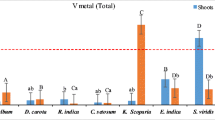
Mean concentrations (mg kg−1) of V, Nb and Ta decreased in the order V (84.7 ± 12.5) > Nb (37.5 ± 4) > Ta (3 ± 0.8) in the sediment and V (0.6 ± 0.6) > Nb (0.02) > Ta (0.002) in the mangrove plants. Species-specific variability in bioaccumulation factor (V: 0.012–0.035; Nb: 0.001–0.003; Ta: 0.001–0.005), translocation factor (V: 0.5–5.1; Nb: 0.26–7.06; Ta: 0.22–2.56) and enrichment factor (V: 0.008–0.027; Nb: 0.0002–0.001; Ta: 1.0 × 10−5-3.0 × 10−6) indicated different partitioning of Group Va elements within the plant organs and varying degree of mangrove uptake efficiency.
Conclusion
Results showed a general decrease in V, Nb and Ta concentrations with their increasing atomic weight. Their total concentrations in plants were related to the degree of enrichment of substrate sediments. The phytoextraction capacity varied amongst mangrove species depending on their CO2 uptake efficiency. Given increased demand for TCEs, results may have important implications for bioremediation processes.






Similar content being viewed by others
References
Amrhein C, Mosher PA, Brown AD (1993) The effects of redox on Mo, U, B, V, and As solubility in evaporation pond soils. Soil Sci 155:249–255
Andrei M, Galateanu B, Hudita A, Costache M, Osiceanu P, Calderon Moreno JM, Drob SI, Demetrescu I (2016) Electrochemical comparison and biological performance of a new CoCrNbMoZr alloy with commercial CoCrMo alloy. Mater Sci Eng C Mater Biol Appl 59:346–355
Baek S, Myeong M, Shin H et al (2017) Superior pre-osteoblast cell response of etched ultrafine-grained titanium with a controlled crystallographic orientation. Sci Rep 7:44213
Barth G, McDonough WF, Rudnick RL (2000) Tracking the budget of Nb and Ta in the continental crust. Chem Geol 165:197–213
Bennett WW, Lombi E, Burton ED, Johnston SG, Kappen P, Howard DL, Canfield DE (2018) Synchrotron X-ray spectroscopy for investigating vanadium speciation in marine sediment. Limitations and opportunities. J Anal Atom Spectrom 33:1689–1699
Boström K, Fisher DE (1971) Volcanogenic uranium, vanadium and iron in Indian Ocean sediments. Earth Planet Sci 11:95–98
Bowen HJM (1979) Environmental chemistry of the elements. Academic Press, London
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Canadian Council of Ministers of the Environment (CCME) (1999) Chapter 7, Canadian Council of Ministers of the Environment, 1999, Winnipeg
Crans DC, Smee JJ, Gaidamauskas E, Yang L (2004) The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev 104:849–902
Da Silva JF, Williams RJP (2001) The biological chemistry of the elements: the inorganic chemistry of life. Oxford University Press, Oxford, UK
Datta DK, Subramanian V (1997) Texture and mineralogy of sediments from the Ganges-Brahmaputra-Meghna river system in the Bengal Basin, Bangladesh and their environmental implications. Environ Geol 30:181–188
Divine KK, Goering PL (2004) Tantalum, chap. 21 of. In: Merian E, Anke M, Ihnat M, Stoeppler M (eds) Elements and their compounds in the environment—occurrence, analysis and biological relevance, 2nd edn. Wiley-VCH Verlag GmbH, Weinheim, pp 1087–1097
Echevarria G, Morel JL, Leclerc-Cessac E (2005) Retention and phytoavailability of radioniobium in soils. J Environ Radiol 78:343–352
Edwards R, Lepp NW, Jones KC (1995) Antimony and vanadium. In: Alloway BJ (ed) Heavy metals in soils, 2nd edn. Blackie Academic & Professional, Glasgow, pp 346–352
Espejo W, Kitamura D, Kidd KA, Celis JE, Kashiwada S, Galbán-Malagón C, Barra R, Chiang G (2018) Biomagnification of tantalum through diverse aquatic food webs. Environ Sci Technol Lett 5:196–201
Filella M (2017) Tantalum in the environment. Earth-Sci Rev 173:122–140
Filella M, Rodushkin I (2018) A concise guide for the determination of less-studied technology-critical elements (Nb, Ta, Ga, In, Ge, Te) by inductively coupled plasma mass spectrometry in environmental samples. Spectrochim Acta Part B Atom Spect 141:80–84
Firdaus ML, Norisuye K, Nakagawa Y, Nakatsuka S, Sohrin Y (2008) Dissolved and labile particulate Zr, Hf, Nb, Ta, Mo and W in the Western North Pacific Ocean. J Oceanogr 64:247–257
Frohne T, Diaz-Bone RA, Du Laing G, Rinklebe J (2015) Impact of systematic change of redox potential on the leaching of Ba, Cr, Sr, and V from a riverine soil into water. J Soils Sediments 15:623–633
Garcia-Jimenez A, Trejo-Tellez LI, Guillen-Sanchez D, Gomez-Merino FC (2018) Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS One 13:e0201908
Garrett RG, Drew LJ, Sutphin DM (2005) Estimating soil geochemistry from 871 stream sediment geochemistry. In: GIS and spatial analysis: proc. 2005 Ann.Conf. 872 international association for mathematical geology (IAMG), 1: 452–45
Garzanti E, Vezzoli G, Ando S, France-Lanord C, Singh SK, Foster G (2004) Sand petrology and focused erosion in collision orogens: the Brahmaputra case. Earth Planet Sci Lett 220:157–174
Gerzabek MH, Mohamad SA, Muck K, Horak O (1994) 60Co, 63Ni and 94Nb soil –to-plant transfer in pot experiments. J Environ Radiol 25:205–212
Goering PL, Ziegler TL (2004) Niobium (Nb) (columbium), chap. 19 of. In: Merian E, Anke M, Ihnat M, Stoeppler M (eds) Elements and their compounds in the environment—occurrence, analysis and biological relevance, vol 2, 2nd edn. Wiley-VCH Verlag GmbH, Weinheim, pp 1039–1046
GOST (State Standard) (1985) 17.4.4.02–84: Nature protection. Soils. Methods of sampling and preparation of material for chemical, bacteriological, and helminthological analysis. (In Russian)
Gough LP, Severson RC, Shacklette HT (1988) Element concentrations in soils and other surficial materials of Alaska. US Geol Survey Prof Pap 1458:53
Gustafsson JP (2019) Vanadium geochemistry in the biogeosphere–speciation, solid-solution interactions, and ecotoxicity. Appl Geochem 102:1–25
Henderson P (2013) Rare earth element geochemistry, vol 2. Elsevier Science Publishers, Amsterdam
Hope BK (2008) A dynamic model for the global cycling of anthropogenic vanadium. Glob Biogeochem Cycles 22(4):GB4021
Huang J-H, Huang F, Evans L, Glasauer S (2015) Vanadium: global (bio)geochemistry. Chem Geol 417:68–89
Jasy JB, Rahman MJJ, Yeasmin R (2010) Sand petrology of the exposed bar deposits of the Brahmaputra-Jamuna River, Bangladesh: implications for provenance. Bangladesh Geosci J 16:1–22
Jha PK, Vaithiyanathan P, Subramanian V (1993) Mineralogical characteristics of the sediments of a Himalayan river: Yamuna River-a tributary of the Ganges. Environ Geol 22:13–20
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer-Verlag, Berlin, 550 p
Kabata-Pendias A, Pendias H (2007) Trace elements in soils and plants © 2001 by CRC Press LLC Washington
Linnen R, Trueman DL, Burt R (2014) Tantalum and niobium. In: Gunn G (ed) Critical metals handbook. Wiley, Oxford
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34:321–342
Mandal SK, Majumder N, Chowdhury C, Ganguly D, Dey M, Jana TK (2012) Adsorption kinetic control of As (III and V) mobilization and sequestration by mangrove sediment. Environ Earth Sci 65:2027–2036
Mandal SK, Ray R, González AG, Mavromatis V, Pokrovsky OS, Jana TK (2019) State of rare earth elements in the sediment and their bioaccumulation by mangroves: a case study in pristine islands of Indian Sundarban. Environ Sci Pollut Res Int 26:9146–9160
Marchand C, Fernandez J-M, Moreton B, Landi L, Lallier-Vergès E, Baltzer F (2012) The partitioning of transitional metals (Fe, Mn, Ni, Cr) inmangrove sediments downstream of a ferralitised ultramafic watershed (New Caledonia). Chem Geol 300-301:70–80
Marchand C, Fernandez J-M, Moreton B (2016) Trace metal geochemistry in mangrove sediments and their transfer to mangrove plants (New Caledonia). Sci Total Environ 562:216–227
Martin JM, Meybeck M (1979) Elemental mass-balance of material carried by major world rivers. Mar Chem 7:173–206
McDonough WF, Sun S (1995) The composition of the earth. Chem Geol 120:223–253
Morrison JF, Cleland WW (1983) Lanthanide ATP complexes determination of their dissociation constants and mechanism of action as inhibitors of yeast hexo kinase. Biochemistry-US 22:5507–5513
Moskalyk RR, Alfantazi AM (2003) Processing of vanadium: a review. Miner Eng 16:793–805
Naidenov M, Travesi A (1977) Nondestructive neutron activation analysis of Bulgarian soils. Soil Sci 124:152
Nandy P (Datta), Ghose M (2001) Photosynthesis and water-use efficiency of some mangroves from Sundarbans, India. Journal of Plant Biology 44(4):213–219
Noël V, Marchand C, Juillot F, Ona-Nguema G, Viollier E, Marakovic G, Olivi L, Delbes L, Gelebart F, Morin G (2014) EXAFS analysis of iron cycling in mangrove sediments downstream a lateritized ultramafic watershed (Vavouto Bay, New Caledonia). Geochim Cosmochim Acta 136:211–228
Pachadzhanov DN (1963) Geochemistry of niobium and tantalum in clays. Geokhimiya 10:930–942
Plank T, Langmuir CH (1998) The chemical composition of subducting sediment and its consequences for the crust and mantle. Chem Geol 145:325–394
Qian Y, Gallagher FJ, Feng H, Wu M, Zhu Q (2014) Vanadium uptake and translocation in dominant plant species on an urban coastal brownfield site. Sci Total Environ 476:696–704
Rahman MJJ, Pownceby MI, Md. Rana S (2020) Occurrence and distribution of valuable heavy minerals in sand deposits of the Jamuna River, Bangladesh. Ore Geol Rev 116:103273
Rankama K (1948) On the geochemistry of niobium: Acad. Sci. Fennicae Annales, ser. A, III. Geol Geog 13:1–57
Ray R, Ganguly D, Chowdhury C, Dey M, Das S, Dutta MK, Mandal SK, Majumder N, De TK, Mukhopadhyay SK, Jana TK (2011) Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmos Environ 45:5016–5024
Ray R, Chowdhury C, Majumdar N, Dutta MK, Mukhopadhyay SK, Jana TK (2013) Improved model calculation of atmospheric CO2 increment in affecting carbon stock of tropical mangrove forest. Tellus B 65:18981
Salminen R, Batista MJ, Bidovec M, Demetriades A, Vivo D et al (2005) Geochemical atlas of Europe. Part 1: background information, methodology and maps. Geological Survey of Finland, Espoo, 526 pages
Sánchez-Rodriguez I, Huerta-Díaz MA, Choumiline E, Holguín-Quiñones O, Zertuche-González JA (2001) Elemental concentrations in different species of seaweeds from Loreto Bay, Baja California Sur, Mexico: implications for the geochemical control of metals in algal tissue. Environ Pollut 114:146–160
Schlesinger WH, Klein EM, Vengosh A (2017) Global biogeochemical cycle of vanadium. PNAS 114(52):E11092–E11100
Schulz KJ, Piatak NM, Papp JF (2017) Niobium and tantalum, chap. M of In: Schulz KJ, DeYoung JH, Jr Seal RR II, Bradley DC (eds) Critical mineral resources of the United States—economic and environmental geology and prospects for future supply: U.S. Geological Survey Professional Paper 1802, p M1– M34
Schwela, Ulric (2011) T.I.C. statistics and transport project: Tantalum-Niobium International Study Center Bulletin, no. 145, p. 2–8
Shacklette HT, Boerngen JG (1984) Element concentrations in soils and other surficial materials of the conterminous United States. US Government Printing Office, Washington DC, USA USGS Professional Paper 1270
Sheppard SC, Long JM, Sanipelli B (2010) Plant/soil concentration ratios for paired field and garden crops, with emphasis on iodine and the role of soil adhesion. J Environ Radiol 101:1032–1037
Sheppard SC, Sohlenius G, Omberg L-S, Borgiel M, Grolander S, Nordén S (2011) Solid/liquid partition coefficients (Kd) and plant/soil concentration ratios (CR) for selected soils, tills and sediments at Forsmark, Svensk Kärnbränslehantering AB Swedish Nuclear Fuel and Waste Management Co Box 250, SE-101 24 Stockholm, ISSN 1402-3091, SKB R-11-24
Shiller AM, Mao LJ (2000) Dissolved vanadium in rivers: effects of silicate weathering. Chem Geol 165(1–2):13–22
Singh SK, France-Lanord C (2002) Tracing the distribution of erosion in the Brahmaputra watershed from isotopic compositions of stream sediments. Earth Planet Sci Lett 202:645–662
Smith DB, Cannon WF, Woodruff LG, Solano F, Kilburn JE, Fey DL (2013) Geochemical and mineralogical data for soils of the conterminous United States: US Geological Survey Data Series 801, 19 p
Srinivasan NR (1957) Tantalum & Niobium Minerals of India. In: Non-ferrous metal industry in India. NML, Jamshedpur, pp 25–29
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell, Oxford, 312 pp
Telfeyan K, Breauxa A, Kimc J, Cablec JE, Kolkera AS, Grimmd DA, Johannesson KH (2017) Arsenic, vanadium, iron, and manganese biogeochemistry in a deltaic wetland, southern Louisiana, USA. Mar Chem 192:32–48
Ure AM, Bacon JR (1978) Comprehensive analysis of soils and rocks by spark-source mass spectrometry. Analyst 103:807
Ure AM, Bacon JR, Berrow ML, Watt JJ (1979) The total trace element content of some Scottish soils by spark source mass spectrometry. Geoderma 22:1–23
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59(7):1217–1232
Yang J et al (2014) Leaching characteristics of vanadiuminmine tailings and soils near a vanadium titanomagnetite mining site. J Hazard Mater 264:498–450
Acknowledgements
SKM was supported by University Grant Commission, New Delhi with a minor research project (No. F, PSW-076/13-14, ERO). RR and AGG are indebted to LabexMER International Postdoctoral Program (FNP150009-DOCTRAY), and Laboratoire d’Excellence LabexMer (ANR-10-LABX-19), respectively. We thank V. Mavromatis (GET, Toulouse) for helping in ICP-MS measurements. Authors sincerely thank the Sundarban Biosphere Reserve for giving permission to undertake this study inside the mangrove forest. The Editor and anonymosus Reviwers are greatly acknowledged for their constructive comments and edits. .
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Ray, R., Dutta, B., Mandal, S.K. et al. Bioaccumulation of vanadium (V), niobium (Nb) and tantalum (Ta) in diverse mangroves of the Indian Sundarbans. Plant Soil 448, 553–564 (2020). https://doi.org/10.1007/s11104-020-04450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04450-2