Abstract
Aims
The causal agent of Fusarium wilt in faba bean is Fusarium oxysporum f. sp. fabae (FOF), which significantly reduces the yield in continuous cropping systems. We aimed to evaluate the role of wheat in alleviating Fusarium wilt in faba bean.
Methods
We assessed the effect of wheat on the occurrence of Fusarium wilt in faba bean and analyzed the differences in the root exudates produced by faba bean in monocropping and intercropping systems before and after infection by FOF.
Results
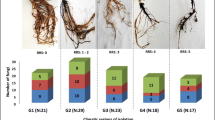
Treatment with FOF increased the incidence and disease index of Fusarium wilt and significantly increased the number of rhizosphere pathogens. Phenolic acids, organic acids, amino acids and sugars exuded from the roots of faba bean increased by 39.1%, 329.0%, 921.7% and 1195.7%, respectively, while intercropping reduced them by 28.04%, 55.69%, 79.10% and 78.75%, respectively. The rhizosphere pathogens of faba bean decreased significantly following intercropping, and the incidence of Fusarium wilt dropped to tolerable levels.
Conclusions
This study suggests that the inoculation with FOF stimulated the exudation of phenolic acids, organic acids, amino acids and sugars from faba bean roots. These compounds were closely related to the changes in the disease resistance of faba bean and could also be used as nutrients to promote pathogen proliferation. After intercropping with wheat, the exudates of faba bean decreased significantly, which may imply that the faba bean recovered from stress, reduced the amount of nutrients needed for pathogen growth, limited the proliferation of pathogens and contributed to the alleviation of Fusarium wilt.




Similar content being viewed by others
References
Boudreau MA (2013) Diseases in intercropping systems. Annu Rev Phytopathol 51:499–519
Chen Y, Zhang F, Tang L, Zheng Y, Li Y, Christie P, Li L (2007) Wheat powdery mildew and foliar N concentrations as influenced by N fertilization and belowground interactions with intercropped faba bean. Plant Soil 291(1–2):1–13
Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommer WB (2004) The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 16(12):3413–3425
Dong Y, Tang L, Zheng Y, W. L. F. (2010) Effects of N application on rhizosphere microflora and fusarium wilt occurrence of intercropped faba bean. Acta Ecol Sin 30(7):1797–1805
Duc G (1997) Faba bean (Vicia faba L.). Field Crop Res 53(1–3):99–109
Emeran AA, Sillero JC, Fernández-Aparicio M, Rubiales D (2011) Chemical control of faba bean rust (Uromyces viciae-fabae). Crop Prot 30(7):907–912
Fabro G, Kovács I, Pavet V, Szabados L, Alvarez ME (2004) Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol Plant-Microbe Interact 17(4):343–350
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92(4):696–717
Gao X, Wu M, Xu R, Wang X, Pan R, Kim HJ, Liao H (2014) Root interactions in a maize/soybean intercropping system control soybean soil-borne disease, red crown rot. PLoS One 9(5):e95031
Hao WY, Ren LX, Ran W, Shen QR (2010) Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil 336:485–497
Haudecoeur E, Planamente S, Cirou A, Tannieres M, Shelp BJ, Morera S, Faure D (2009) Proline antagonizes GABA-induced quenching of quorum-sensing in agrobacterium tumefaciens. Proc Natl Acad Sci 106(34):14587–14592
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res 8:114–124
Lanoue A, Burlat V, Henkes GJ, Koch I, Schurr U, Röse US (2010) De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol 185(2):577–588
Li X, De Boer W, Zhang YN, Ding C, Zhang T, Wang X (2018) Suppression of soil-borne Fusarium pathogens of peanut by intercropping with the medicinal herb Atractylodes lancea. Soil Biol Biochem 116:120–130
Ling N, Raza W, Ma J, Huang Q, Shen Q (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47(6):374–379
Ling N, Zhang W, Wang D, Mao J, Huang Q, Guo S, Shen Q (2013) Root exudates from grafted-root watermelon showed a certain contribution in inhibiting Fusarium oxysporum f. sp. niveum. PLoS One 8(5):e63383
Liu, X. Y., Jin, J. Y., He, P., Liu, H. L., & Li, W. J. (2007). Preliminary study on the relation between potassium chloride suppressing corn stalk rot and soil microorganism characteristics. Plant Nutrition and Fertilizer Science, 2
Lv H, Cao H, Nawaz MA, Sohail H, Huang Y, Cheng F, Kong Q, Bie Z (2018) Wheat intercropping enhances the resistance of watermelon to Fusarium wilt. Front Plant Sci 9
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5(4):359–368
Mattioli R, Falasca G, Sabatini S, Altamura MM, Costantino P, Trovato M (2009) The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol Plant 137(1):72–85
Mohanpuria P, Rana NK, Yadav SK (2007) Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environ Toxicol 22(4):368–374
Momma N, Yamamoto K, Simandi P, Shishido M (2006) Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J Gen Plant Pathol 72(4):247–252
Peng M, Kuc J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82(6):696–699
Ren L, Su S, Yang X, Xu Y, Huang Q, Shen Q (2008) Intercropping with aerobic rice suppressed Fusarium wilt in watermelon. Soil Biol Biochem 40(3):834–844
Ren L, Zhang N, Wu P, Huo H, Xu G, Wu G (2015) Arbuscular mycorrhizal colonization alleviates Fusarium wilt in watermelon and modulates the composition of root exudates. Plant Growth Regul 77(1):77–85
Ren L, Huo H, Zhang F, Hao W, Xiao L, Dong C, Xu G (2016) The components of rice and watermelon root exudates and their effects on pathogenic fungus and watermelon defense. Plant Signal Behav 11(6):e1187357
Stoddard FL, Nicholas AH, Rubiales D, Thomas J, Villegas-Fernández AM (2010) Integrated pest management in faba bean. Field Crop Res 115(3):308–318
Székely G, Ábrahám E, Cséplő Á, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik N, Schmelzer E, Koncz C, Koncz C (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53(1):11–28
Tan S, Yang C, Mei X, Shen S, Raza W, Shen Q, Xu Y (2013) The effect of organic acids from tomato root exudates on rhizosphere colonization of bacillus amyloliquefaciens T-5. Appl Soil Ecol 64:15–22
Tian G, Bi Y, Sun Z, Zhang L (2015) Phenolic acids in the plow layer soil of strawberry fields and their effects on the occurrence of strawberry anthracnose. Eur J Plant Pathol 143(3):581–594
Wang J, Liu H, Ren G (2014) Near-infrared spectroscopy (NIRS) evaluation and regional analysis of Chinese faba bean (Vicia faba L.). Crop J 2(1):28–37
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc London, Ser B 355(1402):1517–1529
Wolfe MS (2000) Crop strength through diversity. Nature 406(6797):681–682
Wu HS, Raza W, Fan JQ, Sun YG, Bao W, Liu DY, Huang QW, Mao ZS, Shen QR, Miao WG (2008) Antibiotic effect of exogenously applied salicylic acid on in vitro soilborne pathogen, Fusarium oxysporum f sp niveum. Chemosphere 74(1):45–50
Wu HS, Wang Y, Zhang CY, Gu M, Liu YX, Chen G, Wang JH, Tang Z, Mao ZH, Shen QR (2009) Physiological and biochemical responses of in vitro Fusarium oxysporum f. sp. niveum to benzoic acid. Folia Microbiol 54(2):115–122
Wu K, Yuan S, Xun G, Shi W, Pan B, Guan H, Shen B, Shen Q (2015) Root exudates from two tobacco cultivars affect colonization of Ralstonia solanacearum and the disease index. Eur J Plant Pathol 141(4):667–677
Xie X, Weiss DJ, Weng B, Liu J, Lu H, Yan C (2013) The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata (S., L.) Yong) roots. Environ Sci Pollut Res 20(2):997–1008
Xu W, Liu D, Wu F, Liu S (2015) Root exudates of wheat are involved in suppression of Fusarium wilt in watermelon in watermelon-wheat companion cropping. Eur J Plant Pathol 141(1):209–216
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166(15):1694–1699
Yang M, Zhang Y, Qi L, Mei X, Liao J, Ding X, Deng W, Fan L, He X, Vivanco J, Li C, Zhu Y, Zhu S (2014) Plant-plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS One 9(12):e115052
Acknowledgments
This work was supported by the Natural Science Foundation of China (31860596, 31560586).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Martin Weih.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lv, J., Dong, Y., Dong, K. et al. Intercropping with wheat suppressed Fusarium wilt in faba bean and modulated the composition of root exudates. Plant Soil 448, 153–164 (2020). https://doi.org/10.1007/s11104-019-04413-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04413-2