Abstract
Background and aims
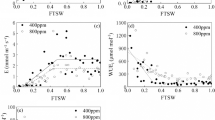
ABA plays an important role in modulating stomatal response to drought and elevated atmospheric CO2 (e [CO2]). This study aimed to investigate the effect of e[CO2] on the response of leaf gas exchange and plant water relations of barley and tomato plants with different endogenous ABA levels to progressive soil drying.
Methods
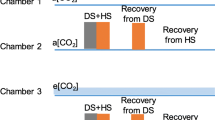
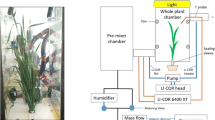
Barley and tomato plants were grown in ambient (a[CO2], 400 ppm) and e[CO2] (800 ppm) and subjected to progressive drought stress. Wild type (WT) genotypes (Steptoe barley and AC tomato) and their ABA-deficient mutants (Az34 barley and flacca) were examined.
Results
e[CO2] sensitized the photosynthetic decline with soil drying. Soil-drying induced stomatal closure was affected by [CO2] in WT genotypes, where e[CO2] sensitized stomatal closure in barley but retarded it in tomato, whereas such effects were absent in mutants. Compared to a[CO2], e[CO2] maintained leaf water potential and improved turgor pressure except in the flacca mutant. For the WT genotypes, the stomata became less sensitive to an increase in leaf ABA concentration ([ABA]leaf) under e[CO2] than a[CO2]; while for both mutants, the stomata was predominately controlled by leaf turgor and not an increase in [ABA]leaf during soil drying.
Conclusion
Endogenous ABA level played an important role in modulating the effect of e[CO2] on stomatal response to soil drying. These findings improve our understanding of the mechanisms of stomatal control in monocot and dicot species responding to a future drier and CO2-enriched environment.









Similar content being viewed by others
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Asch F (2000) Determination of abscisic acid by indirect enzyme linked immuno sorbent assay (ELISA). Technical Report. Laboratory for Agrohydrology and Bioclimatology, Department of Agricultural Science, The Royal Veterinary and Agricultural University, Taastrup, Denmark
Bunce JA (2004) Carbon dioxide effects on stomatal responses to the environment and water use by crops under field conditions. Oecologia 140:1–10
Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang YK, McLachlan DH, Casson S, Isner JC, Wilson I, Neill SJ (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol 25:2709–2716
Chaves MM, Costa JM, Zarrouk O, Pinheiro C, Lopes CM, Pereira JS (2016) Controlling stomatal aperture in semi-arid regions–the dilemma of saving water or being cool? Plant Sci 251:54–64
Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42:55–76
Dodd IC, Theobald JC, Richer SK, Davies WJ (2009) Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalizing shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate. J Exp Bot 60:4029–4039
Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordström M, Azoulay-Shemer T, Rappel WJ, Iba K, Schroeder JI (2016) CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci 21:16–30
Fambrini M, Vernieri P, Toncelli ML, Rossi VD, Pugliesi C (1995) Characterization of a wilty sunflower (L.) mutant. J Exp Bot 46(5):525–530
Faralli M, Williams KS, Han J, Corke FM, Doonan JH, Kettlewell PS (2019) Water-saving traits can protect wheat grain number under progressive soil drying at the meiotic stage: a phenotyping approach. J Plant Growth Regul:1–12
Gray SB, Dermody O, Klein SP, Locke AM, Mcgrath JM, Paul RE, Rosenthal DM, Ruiz-Vera UM, Siebers MH, Strellner R, Ainsworth EA, Bernacchi C, Long SP, Ort DR, Leakey ADB (2016) Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat Plants 2:16132
Haworth M, Killi D, Materassi A, Raschi A, Centritto M (2016) Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front Plant Sci 7:1568
Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 53:1503–1514
Jones HG, Sharp CS, Higgs KH (1987) Growth and water relations of wilty mutants of tomato (Lycopersicon esculentum Mill.). J Exp Bot 38:1848–1856
Kusumi K, Hirotsuka S, Kumamaru T, Iba K (2012) Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot 63:5635–5644
Leakey AD, Bernacchi CJ, Ort DR, Long SP (2006) Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ 29:1794–1800
Li XN, Tan D-X, Jiang D, Liu FL (2016) Melatonin enhances cold tolerance in drought-primed wild-type and abscisic acid-deficient mutant barley. J Pineal Res 61:328–339
Liu FL, Jensen CR, Andersen MN (2003) Hydraulic and chemical signals in the control of leaf expansion and stomatal conductance in soybean exposed to drought stress. Funct Plant Biol 30:65–73
Liu FL, Andersen MN, Jacobsen SE, Jensen CR (2005) Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ Exp Bot 54:33–40
Liu J, Hu TT, Fang L, Peng XY, Liu FL (2019) CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric For Meteorol 268:181–188
Mamatha H, Srinivasa Rao NK, Vijayalakshmi T (2015) Physiological responses of tomato (Lycopersicon esculentum mill) cv. Arka Ashish to elevated atmospheric CO2 under water limiting conditions. Indian J Agric Res 49:299–307
Manzi M, Lado J, Rodrigo MJ, Zacarías L, Arbona V, Gómez-Cadenas A (2015) Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol 56:2457–2466
Martin-Vertedor AI, Dodd IC (2011) Root-to-shoot signalling when soil moisture is heterogeneous: increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf abscisic acid concentration. Plant Cell Environ 34:1164–1175
McAdam SAM, Manzi M, Ross JJ, Brodribb TJ, Gómez-Cadenas A (2016) Uprooting an abscisic acid paradigm: shoots are the primary source. Plant Signal Behav 11:e1169359
Meidner H, Mansfield TA (1968) Physiology of stomata. Bot Gaz 46:62–63
Mulholland BJ, Black CR, Taylor IB, Roberts JA, Lenton JR (1996) Effect of soil compaction on barley (Hordeum vulgare L.) growth: I. possible role for ABA as a root-sourced chemical signal. J Exp Bot 47:539–549
Sagi M, Scazzocchio C, Fluhr R (2002) The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J 31:305–317
Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Biol 52:627–658
Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51:1575–1584
Takahashi F, Suzuki T, Osakabe Y, Betsuyaku S, Kondo Y, Dohmae N, Fukuda H, Yamaguchi-Shinozaki K, Shinozaki K (2018) A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556:235–238
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49:419–432
Tausz-Posch S, Dempsey RW, Seneweera S, Norton RM, Fitzgerald G, Tausz M (2015) Does a freely tillering wheat cultivar benefit more from elevated CO2 than a restricted tillering cultivar in a water-limited environment? Eur J Agron 64:21–28
Tazoe Y, Santrucek J (2015) Superimposed behaviour of gm under ABA-induced stomata closing and low CO2. Plant Cell Environ 38:385–387
Walker-Simmons M, Kudrna DA, Warner RL (1989) Reduced accumulation of ABA during water-stress in a molybdenum cofactor mutant of barley. Plant Physiol 90:728–733
Wei ZH, Du TS, Li XN, Fang L, Liu FL (2018) Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric Water Manag 202:174–182
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2-implications for water-limited environments. Plant Cell Environ 25:319–331
Yan F, Li X, Liu FL (2017) ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ Exp Bot 139:99–104
Zhang F-P, Sussmilch F, Nichols DS, Cardoso AA, Brodribb TJ, McAdam SAM (2018) Leaves, not roots or floral tissue, are the main site of rapid external pressure-induced ABA biosynthesis in angiosperms. J Exp Bot 69:1261–1267
Acknowledgements
This work was partly supported by the Fundamental Research Funds for the Central Universities (2452018063) and National Natural Science Foundation of China (51909220). The technical assistance by Rene Hvidberg Petersen, Britta Garly Henriksen and Lene Korsholm Jørgensen was gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Janusz J. Zwiazek.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenhua Wei and Liang Fang are co-first author
Rights and permissions
About this article
Cite this article
Wei, Z., Fang, L., Li, X. et al. Effects of elevated atmospheric CO2 on leaf gas exchange response to progressive drought in barley and tomato plants with different endogenous ABA levels. Plant Soil 447, 431–446 (2020). https://doi.org/10.1007/s11104-019-04393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04393-3