Abstract
Aims
Separating the effect of resource competition from allelopathy in plants is challenging and it has never been attempted in closely related co-occurring bryophytes. In peatlands, peat mosses (Sphagnum spp.) show niche differentiation along water table level (WTL) gradient. Our aim was to evaluate whether the hummock species, S. magellanicum would be a winner at low WTL due to its allelopathic advantage and the hollow species, S. angustifolium would win by virtue of its superior competitive ability but not of allelopathy at high WTL due to dilution of its allelochemicals.
Methods
We used a nested, field experimental design, with two WTL treatments—low WTL (hummock habitat) and high WTL (hollow habitat)—and three different inter-specific interactions: 1) monoculture; 2) mixed culture without activated charcoal; and 3) mixed culture with activated charcoal added to the neighbor. We measured growth and biochemical traits of the two species and compared the index of relative neighbor effect on each other.
Results
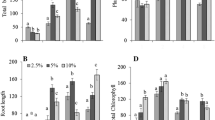
We discovered a trade-off between biomass production (competitive outcome) and phenolic content (allelopathy) in these species. At low WTL, allelopathy of the hummock species is the main mechanism to suppress the hollow species, whereas at high WTL, competition is the main driver to suppress the hummock species.
Conclusions
Competitive advantage in Sphagnum is mediated by both resource competition and allelopathy of the co-occurring species through niche differentiation along a WTL gradient. Unlike vascular plants, Sphagnum mosses can serve as excellent model organisms in studying allelopathic interaction since they bypass the complexity of plant-soil interactions.








Similar content being viewed by others
References
Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301:1377–1380
Bazzaz FA, Nona RC, Coley PD, Pitelka LF (1987) Allocating resources to reproduction and defense. Bioscience 37:58–67
Bengtsson F, Rydin H, Hájek T (2018) Biochemical determinants of litter quality in 15 species of Sphagnum. Plant Soil 425:161–176
Bragazza L (1997) Sphagnum niche diversification in two oligotrophic mires in the southern Alps of Italy. Bryologist 100(4):507–515
Brooker RW (2006) Plant-plant interactions and environmental change. New Phytol 171:271–284
Bu ZJ, Rydin H, Chen X (2011) Direct and interaction-mediated effects of environmental changes on peatland bryophytes. Oecologia 166(2):555–563
Bu ZJ, Chen X, Rydin H, Wang SZ, Ma JZ, Zeng J (2013a) Performance of four mosses in a reciprocal transplant experiment: indication for peatland succession in NE China. J Bryol 35(3):220–227
Bu ZJ, Zheng XX, Rydin H, Moore T, Ma JZ (2013b) Facilitation vs. competition: does inter-specific interaction affect drought responses in Sphagnum? Basic Appl Ecol 14(7):574–584
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2(8):436–443
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Pallini L, Pugnair FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427(6976):731–733
Chiapusio G, Jassey VJ, Hussain MI, Binet P (2013) Evidences of bryophyte allelochemical interactions: the case of Sphagnum. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy. Springer, Berlin Heidelberg, pp 39–54
Chiapusio G, Jassey VEJ, Bellvert F, Comte G, Weston LA, Delarue F, Buttler A, Toussaint ML, Binet P (2018) Sphagnum species modulate their phenolic profiles and mycorrhizal colonization of surrounding Andromeda polifolia along peatland microhabitats. J Chem Ecol 44(12):1146–1157
Clymo RS, Hayward PM (1982) The ecology of Sphagnum. In: smith a.J.E. (ed.) bryophyte ecology, springer Netherlands
Darwin C (1859) On the origin of species by means of natural selection or the preservation of Favoured races in the struggle for life. John Murray, London
Dong X, Dai LM, Shao GF (2005) Forest fire risk zone mapping from satellite images and GIS for Baihe forestry bureau, Jilin, China. J For Res 16(3):169–174
Emery N, Ewanchuk PJ, Bertness MD (2001) Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology 82(9):2471–2485
Ehlers BK, Charpentier A, Grøndahl E (2014) An allelopathic plant facilitates species richness in the Mediterranean garrigue. J Ecol 102:176–185
Eshghi S, Dokhani S, Tafazoli E, Rahemi M, Emam M (2007) Changes in carbohydrate contents in shoot tips, leaves and roots of strawberry (Fragaria× ananassa Duch.) during flower-bud differentiation. Sci Hortic 113:255–260
Fernandez C, Monnier Y, Santonja M, Gallet C, Weston LA, Prévosto B, Saunier A, Baldy V, Bousquet-Mélou A (2013) The impact of competition and allelopathy on the trade-off between plant defense and growth in two contrasting tree species. Front Plant Sci 7:594
Gatti AB, Takao LK, Pereira VC, Ferreira AG, Lima MIS, Gualtieri SCJ (2014) Seasonality effect on the allelopathy of Cerrado species. Braz J Biol 74(3):64S–69S
Gignac LD (1992) Niche structure, resource partitioning, and species interactions of mire bryophytes relative to climatic and ecological gradients in western Canada. Bryologist 95(4):406–418
Granath G, Strengbom J, Rydin H (2010) Rapid ecosystem shifts in peatlands: linking plant physiology and succession. Ecology 91:3047–3056
Grime JP (1979) Plant strategies and vegetation process [M]. John Wiley & Sons, New York
Grime JP, MacKey JML (2002) The role of plasticity in resource capture by plants. Evol Ecol 16(3):299–307
He H, Wang H, Fang C, Lin Z, Yu Z, Lin W (2012) Separation of allelopathy from resource competition using rice/barnyardgrass mixed-culture. PLoS One 7(5):e37201
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Inderjit WDA, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662
Inderjit WLA, Duke S (2005) Challenges, achievements and opportunities in allelopathy research. J Plant Interact 1:69–81
Ingerpuu N, Vellak K (2013) Growth depends on neighbors: experiments with three Sphagnum L. species. J Bryol 35:27–32
Johansson L (1983) Effects of AC in anther cultures. Physiol Mol Plant 59:397–403
John ET, Daniel JW, Mark DS (1983) Contrasting water relations of photosynthesis for two Sphagnum mosses. Ecology 64:1109–1115
Kong CH, Hu F (2001) Planting sensation and its application. China Agriculture Press, Beijing
Koocheki A, Lalegani B, Hosseini S (2013) Ecological consequences of allelopathy. In: Cheema ZA, Farooq M, Wahid A (eds) Allelopathy. Springer Verlag, Berlin Heidelberg, pp 23–38
Laine AM, Juurola E, Hájek T, Tuittila ES (2011) Sphagnum growth and ecophysiology during mire succession. Oecologia 167:1115–1125
Ma J-Z, Bu Z-J, Zheng X-X, Zeng J (2015) Shading enhances the competitive advantage of Sphagnum fallax in a simulation experiment. Mires Peat 16:1–17
Mallik AU, Biswas SR, LC Siegwart C (2016) Belowground interactions between Kalmia angustifolia and Picea mariana: roles of competition, root exudates and ectomycorrhizal association. Plant Soil 403(1–2): 471–483
Mahall BE, Callaway RM (1992) Root communication mechanisms and intracommunity distributions of two Mojave Desert shrubs. Ecology 73(6):2145–2151
Mattson WJ, Julkunen-Tiitto R, Herms DA (2005) CO2 enrichment and carbon partitioning to phenolics: do plant responses accord better with the protein competition or the growth- differentiation balance models? Oikos 111(2):337–347
Mellegård H, Stalheim T, Hormazabal V, Granum PE, Hardy SP (2009) Antibacterial activity of sphagnum acid and other phenolic compounds found in Sphagnum papillosum against food-borne bacteria. Lett Appl Microbiol 49:85–90
Mensuali-Sodi A, Panizza M, Serra G, Tognoni F (1993) Involvement of AC in the modulation of abiotic and biotic ethylene levels in tissue-cultures. Sci Hortic 54:49–57
Meynet P, Hale SE, Davenport RJ, Cornelissen G, Breedveld GD, Werner D (2012) Effect of activated carbon amendment on bacterial community structure and functions in a PAH impacted urban soil. Environ Sci Technol 46:5057–5066
Michael DJ, Anders PM (2002) A survey of the statistical power of research in behavioral ecology and animal behavior. Behav Ecol 14:438–445
Michel P, Burritt DJ, Lee WG (2011) Bryophytes display allelopathic interactions with tree species in native forest ecosystems. Oikos 120:1272–1280
Montenegro G, Portaluppi MC, Salas FA, Diaz MF (2009) Biological properties of the Chilean native moss Sphagnum magellanicum. Biol Res 42:233–237
Mulligan RC, Gignac LD (2002) Bryophyte community structure in a boreal poor fen II: interspecific competition among five mosses. Can J Bot 80(4):330–339
Nilsson MC (1994) Separation of allelopathy and resource competition by the boreal dwarf shrub Empetrum hermaphroditum Hagerup. Oecologia 98:1–7
Novoplansky A (2009) Picking battles wisely: plant behaviour under competition. Plant Cell Environ 32(6):726–741
Parepa M, Schaffner U, Bossdorf O (2012) Sources and modes of action of invasive knotweed allelopathy: the effects of leaf litter and trained soil on the germination and growth of native plants. Neobiota 13:15–30
Piatkowski BT, Shaw AJ (2019) Functional trait evolution in Sphagnum peat mosses and its relationship to niche construction. New Phytol. https://doi.org/10.1111/nph.15825
Pierik R, Mommer L, Voesenek L-ACJ (2013) Molecular mechanisms of plant competition: neighbor detection and response strategies. Funct Ecol 27(4):841–853
Pinto GFS, Kolb RM (2016) Seasonality affects phytotoxic potential of five native species of Neotropical savanna. Botany 94:1–9
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288
Rasmussen S, Wolff C, Rudolph H (1995) Compartmentalization of phenolic constituents in Sphagnum. Phytochemistry 38:35–39
Rice EL (1984) Allelopathy, 2nd edn. Academic Press, New York
Rudolph H, Samland J (1985) Occurrence and metabolism of Sphagnum acid in the cell walls of bryophytes. Phytochemistry 24:745–749
Rydin H (1993) Inter-specific competition between Sphagnum mosses on a raised bog. Oikos 66:413–423
Rydin H (1997) Competition among bryophytes. Adv Bryol 6:135–168
Rydin H, Barber K (2001) Long-term and fine-scale coexistence of closely related species. Folia Geobot 36(1):53–61
San Emeterio L, Damgaard C, Canals RM (2007) Modelling the combined effect of chemical interference and resource competition on the individual growth of two herbaceous populations. Plant Soil 292(1):95–103
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94(4):725–739
Singleton VL, Rossi JA (1964) Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am J Enol Vitic1 6(3):144–158
Soudzilovskaia NA, Graae BJ, Douma JC, Grau O, Milbau A, Shevtsova A, Wolters L, Cornelissen JHC (2011) How do bryophytes govern generative recruitment of vascular plants? New Phytol 190(4):1019–1031
Trewava A, Ballare CL, Trewavas AJ (2009) What is plant behaviour? Plant Cell Environ 32(6):606–616
Tsubota H, Kuroda A, Masuzaki H, Deguchi H (2006) Preliminary study on allelopathic activity of bryophytes under laboratory conditions using the sandwich method. J Hattori Bot Lab 100:517–525
Turetsky MR, Crow SE, Evans RJ, Vitt DH, Wieder RK (2008) Trade-offs in resource allocation among moss species control decomposition in boreal peatlands. J Ecol 96:1297–1305
Van Breemen N (1995) How Sphagnum bogs down other plants. Trends Ecol Evol 10:270–275
Van Kleunen M, Rockle M, Stift M (2015) Admixture between native and invasive populations may increase invasiveness of Mimulus guttatus. Proc R Soc B-Biol Sci 282:20151487
Verhoeven JTA, Liefveld WM (1997) The ecological significance of organochemical compounds in Sphagnum. Acta Bot Neerl 46:117–130
Veteli TO, Mattson WJ, Niemelä P, Julkunen-Tiitto R, Kellomäki S, Kuokkanen K, Lavola A (2007) Do elevated temperature and CO2 generally have counteracting effects on phenolic phytochemistry of boreal trees? J Chem Ecol 33:287–296
Vitt DH, Slack NG (1984) Niche diversification of Sphagnum relative to environmental factors in northern Minnesota peatlands. Can J Bot 62(7):1409–1430
Weißhuhn K, Prati D (2009) Activated carbon may have undesired side effects for testing allelopathy in invasive plants. Basic Appl Ecol 10(2):500–507
Weidenhamer JD, Hartnett DC, Romeo JT (1989) Density-dependent phytotoxicity: distinguishing resource competition and allelopathic interference in plants. J Appl Ecol 26:613–624
Weston LA, Mathesius U (2013) Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol 39(2):283–297
Wright A, Schnitzer SA, Reich PB (2015) Daily environmental conditions determine the competition–facilitation balance for plant water status. J Ecol 103(3):648–656
Yamawo A (2015) Relatedness of neighboring plants alters the expression of indirect defense traits in an extrafloral nectary-bearing plant. Evol Biol 42(1):12–19
Zeng RS, Mallik AZ (2006) Selected ectomycorrhizal fungi of black spruce (Picea mariana) can detoxify phenolic compounds of Kalmia angustifolia. J Chem Ecol 32(7):1473–1489
Acknowledgements
This study was funded by the National Nature Science Foundation of China (No. 41871046 and 41471043), the National Key Research and Development Project (No. 2016YFA0602301 and No. 2016YFC0500407) and Jilin Provincial Science and Technology Development Project (20190101025JH). Håkan Rydin commented on the manuscript. Azim Mallik contributed to this paper during his tenure as a Visiting Professor at the School of Geographical Sciences, Northeast Normal University, Changchun.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Gustavo Gabriel Striker.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 174 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Bu, ZJ., Mallik, A. et al. Resource competition and allelopathy in two peat mosses: implication for niche differentiation. Plant Soil 446, 229–242 (2020). https://doi.org/10.1007/s11104-019-04350-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04350-0