Abstract
Background and aims
Subsoil acidity with a high aluminium (Al3+) soil content inhibits root growth and proliferation of durum wheat (tetraploid AABB, Triticum turgidum) leading to poor nutrient and water uptake. This study evaluated the impact of Al3+-tolerantTaMATE1B allele on root and shoot traits of durum wheat grown in an acidic soil with a high Al3+concentration.
Methods
Two durum wheat lines, Jandaroi–TaMATE1B with the TaMATE1B gene introgressed from Al3+-tolerant bread wheat and Jandaroi–null (a sister line lacking the Al3+-tolerant TaMATE1B allele), were grown in rhizoboxes in a glasshouse. We mapped root growth and proliferation over time and measured shoot traits and grain yield.
Results
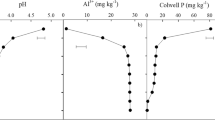
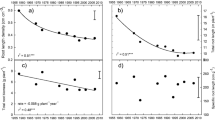
Introgression of the Al3+-tolerant TaMATE1B allele into durum wheat enabled root growth and proliferation below 0.25 m of the soil profile, where the soil pH was low (4.1, CaCl2 extract) with high Al3+ content (16.5 mg kg−1), and increased total root length and biomass at 42 days after sowing (DAS; Z33) by 38.3 and 22%, respectively, relative to the Jandaroi–null. Differences in root growth between the two lines were apparent from tillering stage (Z33) and by 50% anthesis (Z64), respectively. Jandaroi–TaMATE1B had 69.2% greater root biomass, 76.2% greater root length, 5.89% greater leaf area and 18% greater shoot biomass than Jandaroi–null at 50% anthesis (Z64). Time to anthesis and physiological maturity was delayed 6–7 days in Jandaroi–TaMATE1B, compared to Jandaroi–null. Jandaroi–TaMATE1B tended to have relatively greater, but not significantly different, shoot biomass, grain yield and yield components than Jandaroi–null.
Conclusions
Introgression of the Al3+-tolerant TaMATE1B allele into durum wheat enabled root growth and proliferation down an acidic soil profile with a high Al3+ concentration. We assume that in the field where plants need to acquire water at depth differences in above-ground parameters would be amplified.






Similar content being viewed by others
References
Adu MO, Chatot A, Wiesel L, Bennett MJ, Broadley MR, White PJ, Dupuy LX (2014) A scanner system for high–resolution quantification of variation in root growth dynamics of Brassica rapa genotypes. J Exp Bot 65:2039–2048
Anderson WK (2004) Development of the Durum Industry in the Western Region, http://finalreports.grdc.com.au/DAW703
Aziz MM, Palta JA, Siddique KHM, Sadras VO (2016) Five decades of selection for yield reduced root length density and increased nitrogen uptake per unit root length in Australian wheat varieties. Plant Soil 413:181–192
Bona L, Wright RJ, Baligar VC, Matuz J (1993) Screening wheat and other small grains for acid soil tolerance. Landsc Urban Plan 27:175–178
Bona L, Baligar VC, Wright RJ (1995) Soil acidity effects on agribotanical traits of durum and common wheat. In: Date RA, Grundon NJ, Rayment GE, Probert ME (eds) Plant–soil interactions at low pH: principals and management. Kluwer, Dordrecht, pp 425–428
Bozzini A (1988) Origin, distribution, and production of durum wheat in the world. Pages 1-16 in: durum wheat: Chemistry &Technology. Fabriani G, Lintas C, eds. Am Assoc cereal Chem: St Paul, MN
Cai S, Bai GH, Zhang D (2008) Quantitative trait loci for aluminum resistance in Chinese wheat landrace FSW. Theor Appl Genet 117:49–56
Cosic T, Poljak M, Custic M, Rengel Z (1994) Aluminium tolerance of durum wheat germplasm. Euphytica 78:239–243
de O CCE, Dos Santos RR, Pettinelli A (1992) Durum wheat: tolerance to aluminum toxicity in nutrient solution and in the soil. Bragantia 51:69–76
Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993) Aluminum tolerance in wheat (Triticum aestivum L.) I. uptake and distribution of aluminum in root apices. Plant Physiol 103:685–693
Delhaize E, Gruber BD, Ryan PR (2007) The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett 581:2255–2262
Delhaize E, James RA, Ryan PR (2012a) Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytol 195:609–619
Delhaize E, Ma JF, Ryan PR (2012b) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17:341–348
Dessureault-Rompré J, Nowack B, Schulin R, Luster J (2006) Modified micro suction cup/rhizobox approach for the in-situ detection of organic acids in rhizosphere soil solution. Plant Soil 286:99–107
Dieffenbach A, Gottlein AG, Matzner E (1997) In-situ soil solution chemistry in an acid forest soil as influenced by growing roots of Norway spruce (Picea abies [L.] karst.). Plant Soil 192:57–61
Figueroa-Bustos V, Palta JA, Chen Y, Siddique KHM (2018) Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy 8:1–14
Forde BG (2002) The role of long-distance signalling in plant responses to nitrate and other nutrients. J Exp Bot 53:39–43
Gomez K, Gomez A (1984) Statistical procedure for agricultural research. John Wiley & Sons, New York, USA
Haling RE, Simpson RJ, Cukvenor RA, Lambers H, Richardson AE (2011) Effect of soil acidity, soil strength and macropores on root growth and morphology of perennial grass species differing in acid soil resistance. Plant Cell Environ 34:444–456
Han C, Ryan PR, Yan Z, Delhaize E (2014) Introgression of a 4D chromosomal fragment into durum wheat confers aluminium tolerance. Ann Bot 114:134–144
Han C, Zhang P, Ryan PR, Rathjen TM, Yan Z, Delhaize E (2016) Introgression of genes from bread wheat enhances the aluminium tolerance of durum wheat. Theor Appl Genet 129:729–739
Isbell RF (1993) A classification system for Australian soils (third approximation); technical report 2/1993. CSIRO, Townsville, Australia
Joppa LR (1993) Chromosome engineering in tetraploid wheat. Crop Sci 33:908–913
Judd LA, Jackson BE, Fonteno WC (2015) Advancements in root growth measurement technologies and observation capabilities for container-grown plants. Plants 4:369–392
Kneipp J (2008) Durum Wheat Production, NSW Department of Primary Industries, http://www.nvtonline.com.au/wp-content/uploads/2013/03/Crop-Guide-NSW-Durum-Wheat-Production.pdf
Kochian LV, Piñeros MA, Liu J, Magalhães JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminium resistance. Annu Rev Plant Biol 66:571–598
Lemerle D, Cousens RD, Gill GS, Peltzer SJ, Moerkerk M, Murphy CE, Collins D, Cullis BR (2004) Reliability of higher seeding rates of wheat for increased competitiveness with weeds in low rainfall environments. J Agric Sci 142:395–409
Liao M, Palta JA, Fillery IRP (2006) Root characteristics of vigorous wheat improve early nitrogen uptake. Aust J Agric Res 57:1097–1107
Ma HX, Bai GH, Carver BF, Zhou IL (2005) Molecular mapping of a quantitative trait locus for aluminum tolerance in wheat cultivar. Theor Appl Genet 112:51–57
Moustakas M, Yupsanis T, Symeonidis L, Karataglis S (1992) Aluminum toxicity effects on durum wheat cultivars. J Plant Nutr 15:627–638
Nagel KA, Putz A, Gilmer F, Heinz K, Fischbach A, Pfeifer J, Faget M, Blossfeld S, Ernst M, Dimaki C, Kastenholz B, Kleinert AK, Galinski A, Scharr H, Fiorani F, Schurr U (2012) Growscreen-rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Funct Plant Biol 39:891–904
Nagel KA, Bonnett D, Furbank R, Walter A, Schurr U, Watt M (2015) Simultaneous effects of leaf irradiance and soil moisture on growth and root system architecture of novel wheat genotypes: implications for phenotyping. J Exp Bot 66:5441–5452
Palta JA, Watt M (2009) Vigorous crop root systems: form and function for improving the capture of water and nutrients. In applied crop physiology: boundaries between genetic improvement and agronomy; VOSadras DC, Ed.; academic press: San Diego, CA, USA, pp. 309–325
Palta JA, Fillery IRP, Rebetzke GJ (2007) Restricted–tillering wheat does not lead to greater investment in roots and early nitrogen uptake. Field Crops Res 104:52–59
Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M (2011) Large root systems: are they useful in adapting wheat to dry environments? Funct Plant Biol 38:347–354
Pereira JF (2018) Initial root length in wheat is highly correlated with acid soil tolerance in the field. Sci Agric 75:79–83
Pereira JF, Zhou GF, Delhaize E, Richardson T, Zhou MX, Ryan PR (2010) Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Ann Bot 106:205–214
Pfeifer J (2013) Elucidation of root-soil interactions of crops in space and time by establishment and application of novel image based non-invasive root phenotyping methods. PhD. Thesis, ETH Zurich, Zürich, Switzerland
Raman H, Zhang K, Cakir M (2005) Molecular mapping and characterization of ALMT1, the aluminium-tolerance gene of bread wheat (Triticum aestivum L.). Genome 48:781–791
Rao IM, Miles JW, Beebe SE, Horst WJ (2016) Root adaptations to soils with low fertility and aluminium toxicity. Ann Bot 118:593–605
Ryan PR, Raman H, Gupta S, Sasaki T, Yamamoto Y, Delhaize E (2010) Multiple origins of aluminium resistance in hexaploid wheat are derived from Aegilops tauschii and from more recent cis mutations to TaALMT1. Plant J 64:446–455
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37:645–653
Siddique KHM, Belford RK, Tennant D (1990) Root: shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil 121:89–98
Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E (2013) Transposon–mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol 161:880–892
Turner NC, Asseng S (2005) Productivity, sustainability, and rainfall-use efficiency in Australian rainfed Mediterranean agricultural systems. Aust J Agric Res 56(11):1123–1136
Watt M, Kirkegaard JA, Rebetzke GJ (2005) A wheat genotype developed for rapid leaf growth copes well with the physical and biological constraints of unploughed soil. Funct Plant Biol 32:695–706
Watt M, Schneebeli K, Dong P, Wilson IW (2009) The shoot and root growth of brachypodium and its potential as a model for wheat and other cereal crops. Funct Plant Biol 36:960–969
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Acknowledgements
The Department of Education and Training, Government of Australia for Endeavour Research Fellowship award to VP. We thank to Victoria Figueroa-Bustos, Junlin Zheng and Yumika Watanabe for their help with the harvesting and lab work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Jhonathan Ephrath.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16.2 KB)
Rights and permissions
About this article
Cite this article
Pooniya, V., Palta, J.A., Chen, Y. et al. Impact of the TaMATE1B gene on above and below-ground growth of durum wheat grown on an acid and Al3+-toxic soil. Plant Soil 447, 73–84 (2020). https://doi.org/10.1007/s11104-019-04231-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04231-6