Abstract
Aims
Plant roots control many important interactions in the wetland anoxic zone such as carbon deposition, gas exchange, and nutrient dynamics, yet few studies document the vertical depth distribution of fine roots which mediates these interactions.
Methods
Excavated root systems of a wetland sedge and shrub were scanned. Utilizing Root System Analyzer, normalized root length per 5 cm depth interval were quantified for 10 samples of each species. Utilizing bootstrapping, root length per depth interval for each root class was fitted to a density distribution function and matched to water table depth.
Results
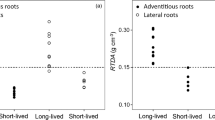
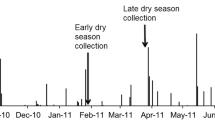
Vertical root length distributions varied by root class, with shrub fine roots constrained to a narrow depth range. Between 1999 and 2010, the interface with the anoxic zone can vary by a factor of four between wet and dry years.
Conclusions
Compared to estimates of root vertical distribution based on biomass, this study indicates a considerably higher portion of the fine roots occur in anoxic soil. Accurately quantifying the spatial distribution of the fine roots, root tips, and other sites associated with exudation is crucial for determining the strength of root-methanogen interactions.









Similar content being viewed by others
References
Abramowitz M, Stegun I (1965) Handbook of mathematical functions with formulas, graphs, and mathematical tables. Washington U.S. Government Print. Off, Washington, DC
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723
Azzalini A (2013) The skew-normal and related families. Cambridge University Press, Cambridge
Bergman I, Svensson BH, Nilsson M (1998) Regulation of methane production in a swedish acid mire by pH, temperature and substrate. Soil Biol Biochem 30(6):729–741
Björk RG, Majdi H, Klemedtsson L, Lewis-Jonsson L, Molau U (2007) Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in northern Sweden. New Phytol 176(4):862–873
Brunner I, Godbold DL (2007) Tree roots in a changing world. J For Res 12(2):78–82
Coles JRP, Yavitt JB (2002) Control of methane metabolism in a forested northern wetland, New York state, by aeration, substrates, and peat size fractions. Geomicrobiol J 19(3):293–315
Dennis JG, Johnson PL (1970) Shoot and rhizome-root standing crops of tundra vegetation at barrow, Alaska. Arct Alp Res 2(4):253–266
Dias ATC, Hoorens B, Van Logtestijn RSP, Vermaat JE, Aerts R (2010) Plant species composition can be used as a proxy to predict methane emissions in wetland ecosystems after land-use changes. Ecosystems 13(4):526–538
Espeleta JF, West JB, Donovan LA (2009) Tree species fine-root demography parallels habitat specialization across a sandhill soil resource gradient. Ecology 90(7):1773–1787
Feddes RA, Hoff H, Bruen M, Dawson T, De Rosnay P, Dirmeyer P, Jackson RB, Kabat P, Kleidon A, Lilly A et al (2001) Modeling root water uptake in hydrological and climate models. Bull Am Meteorol Soc 82(12):2797–2809
Gale MR, Grigal DF (1987) Vertical root distributions of northern tree species in relation to successional status. Can J For Res 17(8):829–834
Gaudinski JB, Torn MS, Riley WJ, Dawson TE, Joslin JD, Majdi H (2010) Measuring and modeling the spectrum of fine-root turnover times in three forests using isotopes, minirhizotrons, and the radix model. Glob Biogeochem Cycles 24(3)
Gill RA, Jackson RB (2000) Global patterns of root turnover for terrestrial ecosystems. New Phytol 147(1):13–31
Greenup AL, Bradford MA, Mcnamara NP, Ineson P, Lee JA (2000) The role of eriophorum vaginatum in CH4 flux from an ombrotrophic wetland. Plant Soil 227(1–2):265–272
Guo DL, Mitchell RJ, Hendricks JJ (2004) Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 140(3):450–457
Guo D, Xia M, Wei X, Chang W, Liu Y, Wang Z (2008) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three chinese temperate tree species. New Phytol 180(3):673–683
Hao X, Zhang R, Kravchenko A (2005) Effects of root density distribution models on root water uptake and water flow under irrigation. Soil Sci 170(3):167–174
Imada S, Taniguchi T, Acharya K, Yamanaka N (2013) Vertical distribution of fine roots of tamarix ramosissima in an arid region of southern Nevada. J Arid Environ 92:46–52
Jackson RB, Schenk HJ, Jobbágy EG, Canadell J, Colello GD, Dickinson RE, Field CB, Friedlingstein P, Heimann M, Hibbard K, Kicklighter DW, Kleidon A, Neilson RP, Parton WJ, Sala OE, Sykes MT (2000) Belowground consequences of vegetation change and their treatment in models. Ecol Appl 10(2):470–483
Jacquemoud S, Verhoef W, Baret F, Bacour C, Zarco-Tejada PJ, Asner GP, François C, Ustin SL (2009) PROSPECT + SAIL models: a review of use for vegetation characterization. Remote Sens Environ 113(SUPPL. 1:S56–S66
Joabsson A, Christensen TR, Wallén B (1999) Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14(10):385–388
Johnson N, Kotz S, Balakrishnan N (1994) Continuous univariate distributions, 2nd ed. edn. Wiley & Sons, New York
Joslin JD, Gaudinski JB, Torn MS, Riley WJ, Hanson PJ (2006) Fine-root turnover patterns and their relationship to root diameter and soil depth in a 14C-labeled hardwood forest. New Phytol 172(3):523–535
King JY, Reeburgh WS (2002) A pulse-labeling experiment to determine the contribution of recent plant photosynthates to net methane emission in arctic wet sedge tundra. Soil Biol Biochem 34(2):173–180
Kirk GJD, Kronzucker HJ (2005) The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Ann Bot 96(4):639–646
Lai DYF, Moore TR, Roulet NT (2014) Spatial and temporal variations of methane flux measured by autochambers in a temperate ombrotrophic wetland. J Geophys Res G Biogeosci 119(5):864–880
Lee HJ, Jeong SE, Kim PJ, Madsen EL, Jeon CO (2015) High resolution depth distribution of bacteria, archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice paddy. Front Microbiol 6(JUN)
Leitner D, Felderer B, Vontobel P, Schnepf A (2014) Recovering root system traits using image analysis exemplified by two-dimensional neutron radiography images of lupine. Plant Physiol 164(1):24–35
Luster J, Göttlein A, Nowack B, Sarret G (2009) Sampling, defining, characterising and modeling the rhizosphere-the soil science tool box. Plant Soil 321(1–2):457–482
Makita N, Hirano Y, Mizoguchi T, Kominami Y, Dannoura M, Ishii H, Finér L, Kanazawa Y (2011) Very fine roots respond to soil depth: biomass allocation, morphology, and physiology in a broad-leaved temperate forest. Ecol Res 26(1):95–104
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari H, Hobbie EA, Iversen CM, Jackson RB et al (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207(3):505–518
Moore TR, Bubier JL, Frolking SE, Lafleur PM, Roulet NT (2002) Plant biomass and production and CO2 exchange in an ombrotrophic bog. J Ecol 90(1):25–36
Murphy M (2009) Getting to the root of the matter: variations in vascular root biomass and production in wetlands and responses to global change. McGill University, Montreal
Murphy MT, Moore TR (2010) Linking root production to aboveground plant characteristics and water table in a temperate bog. Plant Soil 336(1):219–231
Murphy M, Laiho R, Moore TR (2009a) Effects of water table drawdown on root production and aboveground biomass in a boreal bog. Ecosystems 12(8):1268–1282
Murphy MT, McKinley A, Moore TR (2009b) Variations in above-and below-ground vascular plant biomass and water table on a temperate ombrotrophic wetland. Bot 87(9):845–853
Norby RJ, Jackson RB (2000) Root dynamics and global change: seeking an ecosystem perspective. New Phytol 147(1):3–12
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Global Change Biol 141(3):426–442
Ostonen I, Helmisaari H-, Borken W, Tedersoo L, Kukumägi M, Bahram M, Lindroos A-, Nöjd P, Uri V, Merilä P, et al. 2011. Fine root foraging strategies in Norway spruce forests across a european climate gradient. Glob Chang Biol 17(12):3620–3632
Pelletier L, Moore TR, Roulet NT, Garneau M, Beaulieu-Audy V (2007) Methane fluxes from three wetlands in the la Grande rivière watershed, james bay lowland, Canada. J Geophys Res Biogeosci 112(1). https://doi.org/10.1029/2006JG000216
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61(3):167–234
Personeni E, Nguyen C, Marchal P, Pagès L. 2007. Experimental evaluation of an efflux-influx model of C exudation by individual apical root segments. J Exp Bot 58(8):2091–2099
Phillips RP, Erlitz Y, Bier R, Bernhardt ES (2008) New approach for capturing soluble root exudates in forest soils. Funct Ecol 22(6):990–999
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine north american trees. Ecol Monogr 72(2):293–309
Proctor C, He Y (2017) Quantifying root extracts and exudates of sedge and shrub in relation to root morphology. Soil Biol Biochem 114:168–180
Raynaud X (2010) Soil properties are key determinants for the development of exudate gradients in a rhizosphere simulation model. Soil Biol Biochem 42(2):210–219
Rewald B, Raveh E, Gendler T, Ephrath JE, Rachmilevitch S (2012) Phenotypic plasticity and water flux rates of citrus root orders under salinity. J Exp Bot 63(7):2717–2727
Saunders CJ, Megonigal JP, Reynolds JF (2006) Comparison of belowground biomass in C3- and C4-dominated mixed communities in a Chesapeake bay brackish marsh. Plant Soil 280(1–2):305–322
Schenk HJ, Jackson RB (2002) The global biogeography of roots. Ecol Monogr 72(3):311–328
Schnepf A, Leitner D, Klepsch S (2012) Modeling phosphorus uptake by a growing and exuding root system. Vadose Zone J 11(3)
Shaver GR, Billings WD (1975) Root production and root turnover in a wet tundra ecosystem, barrow, Alaska. Ecology 56(2):401–409
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129(3):407–419
Tjoelker MG, Craine JM, Wedin D, Reich PB, Tilman D (2005) Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol 167(2):493–508
Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM (2008) Root anatomy, morphology, and longevity among root orders in vaccinium corymbosum (ericaceae). Am J Bot 95(12):1506–1514
Vogt KA, Vogt DJ, Palmiotto PA, Boon P, O'Hara J, Asbjornsen H (1996) Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 187(2):159–219
Vrugt JA, Van Wijk MT, Hopmans JW, Šimunek J (2001) One-, two-, and three-dimensional root water uptake functions for transient modeling. Water Resour Res 37(10):2457–2470
Wachinger G, Fiedler S, Zepp K, Gattinger A, Sommer M, Roth K (2000) Variability of soil methane production on the micro-scale: spatial association with hot spots of organic material and archaeal populations. Soil Biol Biochem 32(8–9):1121–1130
Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD (2015) Root structural and functional dynamics in terrestrial biosphere models - evaluation and recommendations. New Phytol 205(1):59–78
Watanabe A, Takeda T, Kimura M (1999) Evaluation of origins of CH 4 carbon emitted from rice paddies. J Geophys Res Atmos 104(D19):23623–23629
Watson A, O'Loughlin C (1990) Structural root morphology and biomass of three age-classes of pinus radiata. N Z J For Sci 20(1):97–110
Whiting GJ, Chanton JP (1993) Primary production control of methane emission from wetlands. Nature 364(6440):794–795
Wullschleger SD, Epstein HE, Box EO, Euskirchen ES, Goswami S, Iversen CM, Kattge J, Norby RJ, Van Bodegom PM, Xu X (2014) Plant functional types in earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann Bot 114(1):1–16
Yamaguchi J, Tanaka A, Tanaka A (1990) Quantitative observation on the root system of various crops growing in the field. Soil Sci Plant Nutr 36(3):483–493
Acknowledgments
This work is supported by NSERC Discovery Grant RGPIN-386183 and CFI/ORF #26492 to Dr. Yuhong He, and the Graduate Expansion Fund in the Department of Geography at the University of Toronto Mississauga to Cameron Proctor.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Andrea Schnepf.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Proctor, C., He, Y. Quantifying wetland plant vertical root distribution for estimating the Interface with the anoxic zone. Plant Soil 440, 381–398 (2019). https://doi.org/10.1007/s11104-019-04079-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04079-w