Abstract
Background and aims
Belowground C supply from plant roots may accelerate the decomposition of SOM through the rhizosphere priming effect, but the detailed interaction between substrate quality and rhizosphere C supply is poorly understood. We hypothesize that decomposition of organic matter is enhanced by the combined effect of assimilate C supply to the rhizosphere and substrate amendments.
Methods
Birch trees (Betula pendula) planted in experimental mesocosms; half of these trees were shaded to reduce the supply of assimilate C to roots and ECM fungi. Either 13C-enriched glucose, straw, fungal necromass or C4 biochar were subsequently added to each mesocosm. CO2 efflux derived from substrates were separated from that derived from native SOM and roots based on the isotopic composition of total respired CO2.
Results
The addition of all substrates increased fluxes in both un-shaded and shaded treatments, with greatest total CO2 efflux observed in soils amended with straw. Increases in un-labelled CO2 were observed to be greater in the presence of belowground C supply than in mesocosms with shaded trees.
Conclusions
Turnover of SOM is closely linked to belowground C allocation. The biochemical quality and recalcitrance of litter entering the soil C pool is of critical importance to this priming, as is the interaction with rhizosphere-associated decomposition activity.
Similar content being viewed by others
Introduction
Soil organic matter is the largest C pool in terrestrial ecosystems, and therefore plays an important role in the global C cycle (Ciais et al. 2013; Schlesinger and Bernhardt 2013). In addition, SOM improves the structure and fertility of soils (Six et al. 2000). SOM is mainly composed of a heterogeneous mixture of plant and microbial residues, which vary in chemical structures and decomposition rates (Kögel-Knabner 2002; Simpson and Simpson 2012). Soil CO2 efflux (a combination of CO2 respired by roots and their associated microorganisms (rhizomicrobial respiration) and the decomposition of SOM) is the primary pathway by which terrestrial C returns to the atmosphere. Due to the large amount of global C stored as SOM, changes in SOM decomposition can cause significant changes to the concentrations of atmospheric CO2 (Lal 2004). Hence, it is important to understand the controls and drivers of SOM decomposition in order to forecast terrestrial ecosystem feedbacks, particularly under projected climate change scenarios.
Abiotic factors such as temperature and moisture are regarded as the major drivers of SOM decomposition (Bond-Lamberty and Thomson 2010; Moyano et al. 2012); however, there is increasing evidence that C supply to the rhizosphere, via plant roots, can directly drive SOM decomposition (Subke et al. 2011; Finzi et al. 2015). In addition to the input of litter to soils, plants release labile organic compounds into soils in the form of rhizodeposition, which includes root exudates and sloughed-off root cells (Jones et al. 2009; Pausch and Kuzyakov 2018). These organic compounds may act as energy source for microorganisms, and thereby increase microbial activity. As microbial activity increases, the demand for nutrients also increases which can stimulate the decomposition of SOM. Hence, changes in the productivity of plants can in turn affect rhizodeposition, which may influence the stability of soil C.
Roots of many temperate trees are often heavily colonised by ECM fungi (Lang et al. 2011). ECM fungi supply the host plant with nutrients derived from soils and in return receive up to 22% of photoassimilate C from the host plant (Smith and Read 2002; Hobbie 2006). This C supply allows the fungus to form extensive mycelial networks that can dominate organic horizons (Lindahl et al. 2007; Phillips et al. 2014), and stimulate the mobilization of N in N-limited environments. ECM fungi also have the ability to produce a wide range of enzymes, e.g. β-glucosidase, cellobiohydrolase, N-acetyl glucosaminidase and leucine aminopeptidase, that allow them to mineralize C and N from SOM (Talbot et al. 2008; Brzostek et al. 2015). The majority of ECM fungi are found in mineral horizons, with more decayed litter and humus while saprophytic fungi mainly colonize fresh litter found in the top layers of soil (Rosling et al. 2003; Lindahl et al. 2007). However, the ability of ECM fungi to successfully compete with saprophytic fungi and other soil microorganisms is dependent on a supply of C from host plant roots (Lindahl et al. 2010). The competition for limiting nutrients between these two fungal groups was hypothesized to retard SOM decomposition (Gadgil effect, (Gadgil and Gadgil 1971; Gadgil and Gadgil 1975)). However, studies have shown that ECM fungi stimulate SOM decomposition as a result of C provisions from roots (Brzostek et al. 2015). Experimental reduction in C allocation to ECM fungi through girdling and tree shading results in significant decreases in soil respiration and fungal biomass (Högberg and Högberg 2002; Subke et al. 2004; Hasselquist et al. 2016; Tang et al. 2019), which implies that a reduction in assimilate C alters root respiration but may also reduce the activities of microbes in the rhizosphere, potentially reducing SOM decomposition.
The input of organic substrates into soils can either increase or decrease SOM decomposition via a ‘priming effect’, defined as a short-term change in the decomposition of SOM caused by the input of a substrate, e.g. fertilizers (organic or mineral) or plant residues (Kuzyakov et al. 2000). The rhizosphere priming effect (RPE) is the change in SOM decomposition driven by the presence of plant roots (Kuzyakov 2002; Cheng and Kuzyakov 2005). Compared to soils without roots, a broad range of RPE from 50% reduction to 380% acceleration of SOM decomposition in the presence of roots has been reported (Cheng et al. 2014). Readily accessible C and plant residues may stimulate activities of microbial populations, thereby increasing SOM decomposition as a result of increased production of extracellular enzymes (Phillips and Fahey 2006; Yin et al. 2014). CO2 evolution following the input of labile substrate can be used to classify PE into either a ‘real’ or an ‘apparent’ effect (Blagodatskaya and Kuzyakov 2008). The activation of microbial metabolism and acceleration of microbial biomass C turnover is referred to as ‘apparent’ PE (Blagodatskaya et al. 2007), as observed increases in CO2 flux are not associated with decomposition of SOM. ‘Real’ priming effects require an actual acceleration of SOM decomposition caused by change in microbial community structure and extracellular enzyme production (Blagodatskaya and Kuzyakov 2008). Future predictions of elevated atmospheric CO2 concentrations and higher temperature may increase net primary productivity through CO2 fertilisation (Norby et al. 2005). Increased plant productivity results in higher rhizodeposition and plant litter production (Zak et al. 2011), thereby resulting in a net increase in C stock. However, due to the potential priming effect of increased input of both labile and complex C into soils, the degree to which priming effect offsets this higher C input is uncertain.
The magnitude and direction of priming effects are influenced by nutrient availability, quality or quantity of substrate C. The quality of substrate is related to its susceptibility to microbial uptake and enzymatic degradation, for which the C to N ratio (C:N) and the concentration of recalcitrant fractions such as lignin, phenolics and tannins have been used as proxies (Chen et al. 2014; Wang et al. 2015; Di Lonardo et al. 2017). Substrates of low recalcitrance and a higher availability of C (often considered to be of ‘high quality’) may induce higher PEs than substrates with less available C or more recalcitrant compounds (Blagodatskaya and Kuzyakov 2008). Soil microbial communities can influence the PE, as specific microbial groups (r versus K strategists) preferentially dominate decomposition of labile and recalcitrant C pools (Fontaine et al. 2003). However, our knowledge of the effects of substrate quality, and the interaction with the microbial community on PE is limited, and only a few studies have tested this theory (Chen et al. 2014; Wang et al. 2015). Several studies have investigated the individual effects of C inputs from plants or substrates on organic matter decomposition (Zhang et al. 2013; Luo et al. 2016; Huo et al. 2017), but their interactions have not been widely explored. In a girdling study, (Subke et al. 2004) observed additional efflux in litter-amended, non-girdled plots, which was not significant in litter-amended, girdled plots or non-amended, non-girdled plots. Since organic matter enters the soil environment in different forms (for example, as rhizodeposits, litter or microbial necromass), and may differentially affect the PE, it is important to determine the range of potential effects on both old and recent SOM decomposition in order to predict changes in C storage with different C inputs.
Here, we aim to determine the effect of an altered C supply to roots, and their associated ECM fungi, on the decomposition of a range of substrates, and to assess the decomposition of SOM with the input of 13C-labelled substrates of different qualities. To address these aims, we tested the following hypotheses: (1) Decomposition of organic substrates is accelerated by the supply of assimilate C to the rhizosphere. (2) Decomposition of older native SOM is increased by the combined effect of substrate addition and rhizosphere C supply, (3) Soil priming (increased un-labelled CO2) is greater following the addition of readily available glucose compared to more complex organic substrates (i.e. straw, fungal necromass or biochar).
Materials and methods
Experimental design
Forty experimental mesocosms were constructed using 33 L boxes (71 × 44 × 16.5 cm, Really Useful Products Ltd., Normanton, UK). Mesocosms were assigned into four blocks in a randomised complete block design, each containing a shaded/not-shaded treatment, and a substrate treatment (4 blocks × 2 shading treatments × 5 substrate treatments). Organic-rich mineral soil (pH (H2O) 4.04, C:N 14.9) was collected from the top 15 cm of a mixed broadleaf woodland in Stirling, Scotland, UK (5608′ N, 3054′ W), which was dominated by birch (Betula pendula) and beech (Fagus sylvatica) with little understory vegetation. The soil was a freely draining, brown earth formed from fluvioglacial sands and gravels derived mainly from carboniferous soil in the Dreghorn soil series of UK (National soil map of Scotland). The soil was air-dried, sieved (< 2 mm) and homogenised. A two-year old birch tree was planted in each mesocosm in August 2015, watered and allowed to establish for 8 months. Although relative amount of ECM fungi in the mesocosms were not determined directly, the presence of ECM was confirmed using in-growth nylon mesh bags (6 × 6 cm; 41 μm mesh size, Normesh Ltd., Oldham, UK) filled with sterilized sand (Wallander et al. 2013). These in-growth bags had been used to differentiate between ectomycorrhizal hypha from that of saprotrophs, as ECM fungi are able to grow in sand while saprotrophic fungi can not (Wallander et al. 2001; Ekblad et al. 2013). In spring 2016, collars (10 cm diameter, 2 cm high) were inserted (< 1 cm deep) into all mesocosms, taking care to minimise disturbance to roots. All mesocosms were kept outside, in the grounds of the University of Stirling, Scotland, where the mean annual air temperature is 9.2 °C and the mean annual precipitation is c. 1019 mm (UK Met Office 2017). Mesocosms were not watered to avoid oversaturation as mesocosms were exposed to outdoor precipitation. Rainfall prior to and during the experiment meant that soil moisture conditions were not limiting to plant and microbial functioning.
On 2nd August 2016, nine days prior to the addition of the substrates, 20 trees were shaded to reduce photosynthesis and reduce the supply of C to roots and their fungal symbionts. Shading was achieved by using dark but air-permeable phormisol material (LBS Worldwide Ltd., Lancashire, UK) to cover each tree. This material limited irradiance by at least 90%, whilst allowing air exchange and avoiding excessive rises in air temperature for the shaded trees. Care was also taken to ensure that soils were not covered by shading material in order to minimise changes in soil temperature.
Preparation of 13C-labelled substrates
Four substrates were selected to represent different forms of organic materials with varying structural composition and complexities: glucose, straw, fungal necromass and biochar.
13C-glucose was obtained by diluting 13C-D-glucose (99 atom%. Cambridge Isotope Laboratories, Inc., Andover, USA) with D-glucose of natural isotope abundance (Fisher Scientific UK Ltd., Loughborough, UK) with a dilution factor of 1:20, resulting in an enrichment in 13C of c. 5 atom%.
13C wheat straw (Triticum aestivum) was obtained from wheat grown in a chamber enriched with 10 atom% 13CO2. The straw was rinsed in deionised water 4 times, air dried for several days, then shredded (<2 mm) using a grinder.
Mycelium of the basidiomycete Hebeloma crustuliniforme UP184 was grown on agar in Petri dishes containing 1/10 Modified Melin Norkans (MMN) growth medium (Marx 1969), with glucose reduced from 10 g to 1 g. Based on the result of a pilot study to determine the medium composition that supports the most rapid fungal growth, a modified MMN growth medium containing 10 g of malt extract (instead of 3 g) was selected for growing 13C-labelled fungal biomass. In the liquid growth medium (100 cm3), the fungal biomass was labelled by replacing 20% of the 12C-glucose with 99 atom% 13C-glucose (CK Isotopes Ltd., Leicester, UK). Flasks were incubated at 20 °C for 30 days, or until filled with mycelia. Mycelium was harvested from each flask, and rinsed with deionized water 4–5 times to remove any remaining media, air-dried and frozen until further use, when the mycelium was homogenised using a grinder (< 2 mm).
Biochar was obtained from the pyrolysis of Miscanthus (a C4 species) at 450 °C and subsequently ground (<2 mm). The δ13C values of all substrates were confirmed at Life Sciences Mass Spectrometry Facility (CEH, Lancaster, UK) using an automated elemental analyser NA1500 (Carlo Erba, Milan, Italy) coupled to an Isotope Ratio Mass-Spectrometer (Dennis Leigh Technology Ltd., Keele, UK).
Each treatment was represented in all blocks to ensure that all treatments were exposed to similar conditions. The four substrates with distinct 13C signatures were applied as dry powder to the collars on 11th August 2016. Substrates were applied to collars at the rate of approximatly 3 mg C g−1 soil except for biochar, which was applied at rate of 4.7 mg C g−1 soil (collar area = 79 cm2, depth = 2 cm). The isotopic signatures and the exact amount of the added substrates are presented in Table 1. Substrates were thoroughly mixed into the top 2 cm of the soil within each collar, while control collars were also mixed although no substrate was added. 250 cm3 of water was subsequently added to all treatments including control treatments.
Soil respiration and isotopic measurements
Soil respiration measurements were carried out using a portable EGM-4 infrared gas analyser (PP Systems, Amesbury, MA, USA) attached to a 15 cm diameter custom-built respiration chamber with a headspace volume of approximately 2300 cm3, inserted gently into soil (< 1 cm). Respiration rates were derived from linear rise in CO2 concentration within the closed chamber system over a period of two minutes. Sampling for isotopic composition of evolved CO2 was carried out at 2.5 and 5 h (for glucose, straw and control treatments only), 1, 3, 7, 15 and 30 d after substrate addition. On each sampling occasion, custom-built cylindrical PVC chambers (15 cm diameter, 5 cm high) were placed on the soil, ensuring a good seal between soil and chamber. CO2 was allowed to accumulate in these chambers for 15 min, resulting in concentrations of around 1886 ± 92.3 (mean ± 1SE) ppm of CO2. Gas samples (20 ml) were then collected using 20 ml syringes through a septum in the chamber and injected under pressure into previously evacuated borosilicate exetainers (12 ml; Labco Ltd., UK) for isotopic analysis. The concentration of CO2 in all gas samples was obtained using a gas chromatograph, GC-MS (Hewlett Packard 5890) coupled to a flame ionization detector. Isotopic measurements for δ13C values of the gas samples were measured at the Life Sciences Mass Spectrometry Facility (CEH, Lancaster, UK) using an Isoprime Tracegas Preconcentrator coupled to an IsoPrime Isotope Ratio Mass Spectrometer (Elementar UK Ltd., Stockport, UK) at an analytical precision of ±0.17‰.
Soil sampling and analysis
Soil samples were collected to a depth of 5 cm from each collar and stored in sealed plastic bags at 4 °C until processing for soil microbial biomass C, which was determined using the fumigation extraction method (Vance et al. 1987; Joergensen 1996). Fresh soil was passed through a 2 mm sieve from which 5 g each was weighed into two glass jars (20 ml). One jar was fumigated in a desiccator containing ethanol-free CHCl3 and water (to avoid drying) for 24 h and evacuated using a vacuum pump, while the other jar was not fumigated. After fumigation for 24 h, CHCl3 was removed from the soil by evacuating the desiccator three to four times using a vacuum pump. Both the fumigated and non-fumigated soils were extracted with 20 ml 0.5 M K2SO4 (1:4 w/v; soil:extractant) and the mixture shaken for 30 min at 300 rpm, before being filtered through Whatman no. 42 filter papers (pore size: 2.5 μm). Soil microbial biomass C was determined based on the difference between fumigated and non-fumigated soils using the kec factor of 0.45 (Wu et al. 1990). Total organic C (TOC) and total organic N (TON) content of the filtrates were determined using a TOC – VCSN analyzer (Shimadzu Corporation, Kyoto, Japan).
Calculations and statistical analysis
Isotopic abundance of CO2 collected from chambers was corrected for atmospheric CO2 using the isotopic ratio and concentration of atmospheric CO2 present in the chambers at the start of measurements. A two end-member linear mixing model was formulated to partition total soil flux (FTotal) into substrate-derived CO2 (FSub) and ‘soil-derived’ CO2 (FSoil) based on the 13C isotopic abundance between the substrates and the soil using the following mass balance equation:
Where FT, FSub and FSoil are the total CO2 flux, substrate derived CO2 and soil derived CO2 respectively, and δT, δSub and δSoil are the δ13C isotopic signatures for the total flux, substrates and soil, respectively. In this experimental setup, CO2 derived from sources other than the added substrate (i.e. both heterotrophic and rhizomicrobial respiration) are considered as one isotopic pool (indicated by “soil”), while the suffix “sub” represents the glucose, straw, fungal necromass or biochar treatments. The proportion of substrate in the total flux was calculated as:
where δT, is δ13C obtained from CO2 samples collected from all soil collars after correction for atmospheric CO2. δSub is δ13C of the labelled- glucose, straw, fungal necromass or biochar, while δNS is δ13C obtained from CO2 samples collected from control soils where no substrate was added. The CO2 derived from substrates was calculated as:
and the standard error for the proportion of substrate flux contribution (fSub) was calculated according to the method by Phillips and Gregg (2001), which accounts for variabilities in both the mixture (δ13CTotal) and the sources (δ13CSub and δ13CSoil) as:
where σ2 represent the square of the standard errors of the mean isotopic signatures for the components as indicated by the suffixes.
Priming effects were calculated in two categories. First, priming of soil C decomposition in un-shaded or shaded treatments induced by the addition of substrates was calculated as the difference of the CO2 produced from control soils without substrate amendment from CO2 derived from sources other than the substrate added (Eq. 6):
Where amendedFSoil is the ‘soil-derived’ CO2 in soils amended with biochar, straw, fungal necromass or glucose of shaded or un-shaded treatments determined by FSoil = FT– Fsub; and NSFSoil is the ‘soil-derived’ CO2 in soils without substrate addition for the respective light condition.
Second, priming of added substrate decomposition induced by rhizosphere input was calculated as the difference of substrate-derived CO2 in shaded conditions from substrate-derived CO2 from soils in un-shaded conditions. The priming effect of substrate decomposition (PESub) induced by rhizosphere C input was calculated as:
where unshadedFSub is the substrate-derived CO2 in soils that receive autotrophic inputs from roots of un-shaded trees with added substrate, and shadedFSub is the substrate-derived CO2 in soils without root input from shaded trees with added substrate.
Effects of shading and substrate additions on total soil CO2 efflux, soil-derived CO2, substrate-derived CO2, PE and soil microbial biomass C were analysed using two-way analysis of variance (ANOVA) following linear mixed effect models, where ‘mesocosm’ nested within blocks and sampling dates were assigned as random factors. Data were log-transformed when necessary to meet the assumptions of normal distribution. When statistical significance was observed, Tukey post-hoc tests were used for pairwise comparisons at significance level of 0.05. All analyses were carried out using RStudio v0.99.903.
Results
Soil respiration
Prior to tree shading, there was no significant difference between CO2 efflux from soil collars assigned as un-shaded and shaded treatments (Fig. 1). Nine days after shading, soil respiration decreased significantly by 63% relative to un-shaded treatments (p < 0.001) and this reduction in CO2 efflux was significant for all subsequent sampling dates. Average soil CO2 efflux from un-shaded mesocosms (2.39 ± 0.38 μmol m−2 s−1) was significantly greater than that of shaded mesocosms (0.78 ± 0.08 μmol m−2 s−1) for the period after shading (P < 0.001, Table 2)). The relative contribution of root-derived CO2 (rhizomicrobial respiration) to the total cumulative soil CO2 efflux was 67%, calculated as differences between un-shaded and shaded treatments in control (un-amended) soils.
Average soil CO2 efflux for the period of 16th May 2016 to 10th September 2016 in mesocosms without substrates added (control treatments). Soils were watered on 18th July 2016 prior to measurement on 19th July 2016 due to excessive dryness in the mesocosms. Trees were shaded on 2nd August 2016, indicated by the red line. Error bars represent ±1 SE (n = 4). There was no significant difference between shading treatments prior to shading (P = 0.6274) but significantly different after shading (P < 0.001, paired t-test)
The addition of substrates to soils significantly increased total CO2 efflux (P < 0.001) from both un-shaded and shaded treatments throughout the sampling period of 30 days, relative to controls without substrate (Figs. 2 and 3). While shading and substrate addition significantly affected total soil CO2 efflux, there was no significant interaction between these two fixed effects (Table 2). Total soil CO2 efflux increased significantly following the addition of straw, fungal necromass and biochar in un-shaded treatments. In shaded treatments on the other hand, the addition of biochar significantly increased soil CO2 flux (P < 0.05, Tukey-post hoc test) while glucose, straw and fungal necromass had no effect on total soil CO2 efflux.
Soil CO2 efflux in un-shaded (open shapes) and shaded (filled shapes) treatments derived from the added 13C labelled substrate derived CO2 (a & b) and un-labelled soil CO2, (c & d) for the duration of 30 days after the addition of glucose, straw, fungal necromass and biochar. Values denote mean ± 1 SE (n = 4)
Partitioning of total CO2 efflux into CO2 derived from root and heterotrophic (“Soil derived CO2”) and from the addition of glucose, straw, fungal necromass and biochar (“Substrate-derived CO2”) in un-shaded and shaded treatments. Control treatments received water only. Error bars are 1SE of mean (n = 4). Different letters indicate significant interactions between shading and substrate factors (P < 0.05, Tukey post hoc) for soil-derived CO2 efflux (small letters) and substrate-derived CO2 (capital letters)
Increases in δ13C of soils were observed within 5 h of glucose and straw addition, and 24 h of fungal necromass addition when the first measurements were taken (Fig. 4). The respiration of glucose was highest in the first 7 days after its addition in both shaded and un-shaded treatments after which decomposition of glucose declined, with a higher decomposition of glucose in the shaded treatment than the un-shaded treatment. The decomposition of straw and fungal necromass were greater in shaded treatments and continued throughout the sampling period while in un-shaded treatments, both, decomposition of straw and fungal necromass was highest on day 7. 13C values in biochar treated soils were not different from those in control mesocosms that received only water.
Source-partitioning and priming effects
CO2 efflux derived from added substrate was significantly different among substrates (P < 0.001) but not between shading treatments (Table 2 and Fig. 3). In both un-shaded and shaded treatments, the majority of glucose-derived CO2 evolved within seven days of glucose addition, with higher CO2 flux rates from glucose-amended mesocosms with shaded trees (Fig. 2a, b). For the period of 30 days after substrate addition, CO2 evolved from other sources other than the added substrate ranged between 3.40 ± 0.74 and 7.56 ± 2.21 μmol m−2 s−1 in un-shaded treatments while it ranged between 1.40 ± 0.15 and 1.65 ± 0.31 μmol m−2 s−1 in shaded treatments. Adding straw to un-shaded mesocosms resulted in ‘soil-derived’ CO2 that was approximately three times higher than control mesocosms (un-shaded, no substrates) and about 5 times that produced from the corresponding shaded treatment (shaded, straw added; Fig. 3). In un-shaded mesocosms, un-labelled CO2 efflux increased immediately following the addition of straw by up to four times relative to un-shaded control mesocosms and the increases persisted until the end of sampling (Fig. 2c). For the shaded treatments, the addition of glucose, straw, fungal necromass and biochar increased un-labelled CO2 during the first 3 days only (Fig. 2d). Glucose amended soils produced the least CO2 from soil in both un-shaded and shaded conditions, but more CO2 derived from the added substrate.
No significant priming of substrate decomposition by the supply of C to the rhizosphere (PESub) as calculated by the difference between substrate derived CO2 in un-shaded and shaded treatments was observed (Fig. 3). However, increases in un-labelled CO2 (priming effects) after the addition of all substrates were observed in both shaded and un-shaded trees (Figs. 5 and 6). These priming effects were not different between soils with un-shaded and shaded trees, with the exception of straw treatments where the un-shaded treatment was significantly higher than the shaded treatment (P < 0.01). The addition of glucose generated the lowest PE over the experimental period in the shaded treatments (88%) and in the un-shaded treatment (61%). The PE was significantly higher immediately after the addition of 13C-labelled straw and glucose (5 h) and fungal necromass and biochar (1 d) in both shading treatments (Fig. 5). Following this initial flush, no significant PE was observed until 15 days after substrate additions in un-shaded treatments.
Microbial biomass carbon
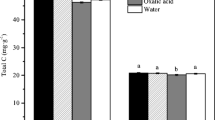
At the end of the sampling period, soil microbial biomass C was generally higher in un-shaded treatments than in shaded treatments (P < 0.05). However, there was no overall significant difference in soil microbial biomass C among substrates, nor between the interaction of shading and substrates (Fig. 6).
Discussion
The alteration of rhizosphere C supply through shading reduced total soil CO2 efflux across all substrate treatments. The result also provides evidence that the combined effects of rhizosphere C supply and substrate amendments can increase decomposition of native SOM, the magnitude of which is dependent on the quality of the amendment. Tree shading reduced soil CO2 efflux by about 67% for the duration of the experiment suggesting C supply to roots and their associated ECM fungi was strongly reduced, which suggests that assimilate C flux to roots is a major driver of soil CO2 efflux. It is commonly reported that roots use their carbohydrate reserves after assimilate C supply is altered through girdling, trenching, defoliation or drought, thereby delaying the reduction in respiration rates and underestimating autotrophic respiration (Högberg et al. 2001; Díaz-Pinés et al. 2010; Hasibeder et al. 2015). However, plant age influences the availability of root carbohydrate reserves (Bahn et al. 2006); hence, in contrast to the mature forests reported in other studies, it is likely that the root C reserves were rapidly used up following shading in the young trees (3 years old) used in this study, resulting in rapid decline in root respiration. Consistent with our finding, a recent study reported that rhizosphere respirations in the presence of soybean and sunflower were positively related with light intensity, as lower rhizosphere respirations were observed from two days of subjecting the plants to varying degrees of shading (Tang et al. 2019). Shading is potentially an effective partitioning method with a number of benefits compared to root exclusion methods (such as trenching and other physical separation techniques). Some of these methods may significantly underestimate the contribution of roots due to limitations such as the physical disturbance to the soil structure and decomposition of decayed roots (Subke et al. 2006).
The addition of glucose, straw, fungal necromass and biochar, representing organic matter of varying structural complexities, resulted in increased microbial metabolic activities as indicated by higher CO2 fluxes from soils of both shaded and un-shaded trees. As shown by the excess 13C in soil CO2 efflux, glucose, straw and fungal necromass were decomposed in both shaded and un-shaded treatments, while biochar was not decomposed in either shading treatments. The lack of biochar decomposition was likely because biochar, being a relatively recalcitrant substrate (Cross and Sohi 2011; Schmidt et al. 2011) is not readily available for microbial metabolism. Both, lack of, and decomposition of biochar have been reported in incubation studies (Zimmerman 2010; Stewart et al. 2013; Cui et al. 2017; Luo et al. 2017a), and the contrasting results may be attributed to differences in the combustion temperature, duration and biomass used for biochar production, which influence its lability (Zimmerman 2010). It is unlikely that the lack of biochar decomposition was due to the narrow difference in the δ13C signature between biochar (−4‰) and the soils (approx. -28‰), as the difference in δ13C values lies the basis for natural abundance isotopic partitioning where C4 plants are grown on C3 soil or vice versa. Moreover, a recent study successfully partitioned soil-derived CO2 from root-derived CO2 by planting C3 trees (approx. -27‰) on C4 soils (−17‰), thereby showing that the >10‰ difference in δ13C values was successful in partitioning CO2 sources (Yin et al. 2018).
The supply of assimilate C to the rhizosphere did not enhance the decomposition of substrates, as we did not observe any difference in the mineralization of glucose, straw, fungal necromass or biochar between shaded and non-shaded treatments. Previous studies that investigated the interaction between root activity and litter decomposition have either reported a positive (Subke et al. 2004; Subke et al. 2011; Trap et al. 2017) or negative (Gadgil and Gadgil 1971; Averill et al. 2014) effect of roots on litter decomposition. Since roots and ECM were present in both un-shaded and shaded treatments, it is possible that in addition to heterotrophic saprotrophs, ECM fungi contributed to the mineralization of the added substrates. Although, ECM fungi receive their C primarily from host plant photosynthates in return for nutrients, they may also access soil C either from the metabolism of low molecular weight compounds (Talbot et al. 2008) or through decomposition of complex organic compounds (Phillips et al. 2014) by investing in enzymes involved in the mobilization of C (Buée et al. 2005; Buée et al. 2007; Courty et al. 2007).
As flux partitioning using the isotopic composition showed, the addition of fresh substrate increased CO2 flux from sources other than the added substrate. This additional CO2 efflux might have been caused by increased root-derived respiration, increased turnover of soil microbial biomass and/or accelerated SOM decomposition, all of which had similar isotopic compositions. We hypothesise that the additional un-labelled CO2 production was caused by an initial increase in the turnover of microbial biomass C (apparent PE), followed by increased decomposition of SOM (real PE) rather than from higher rhizo-microbial respiration. It is possible that the proliferation of roots and associated mycorrhizal fungi in response to the addition of substrates also contributed to the observed increase in un-labelled CO2 flux. However, the absence of a similar proliferation in shaded treatments suggests that the availability of labile C in the rhizosphere, not root growth causes the observed pattern. In a pot experiment, to test the contribution of ectomycorrhizal roots to the additional un-labelled CO2 released following the addition of 13C-labelled sucrose, Ekblad and Högberg (2000) found no increase in rhizomicrobial respiration with added sugar, corroborating our conclusion that the additional un-labelled CO2 is from organic matter decomposition. However, further confirmation of our assumption is required using a three-source partitioning approach to separate CO2 derived from roots, SOM and substrates.
The rapid flush of un-labelled CO2 efflux from both shaded and un-shaded treatments on or before 24 h was likely due to increased microbial turnover rates, and we propose that real PE involving the decomposition of SOM commenced after 1 day of substrate additions in the forest soil of our mesocosms (Blagodatskaya and Kuzyakov 2008; Blagodatsky et al. 2010). Therefore, this study demonstrates that the addition of complex substrates mostly causes real PE. Most likely due to the production of extracellular enzymes and subsequent co-metabolism of SOM (Blagodatskaya and Kuzyakov 2008; Fontaine et al. 2011; Blagodatskaya et al. 2014). The PE occurred in two stages: short-term apparent PE occurring immediately after substrate addition followed by long-term real PE, which was predicted by the model of Blagodatsky et al. (2010). This temporal dynamic of PE has also been reported by others (Nottingham et al. 2009; Blagodatskaya et al. 2011), and requires confirmation by distinct partitioning of rhizomicrobial respiration from microbial decomposition, whilst also tracing microbial biomass pools with a three-source labelling approach.
Positive priming effects of substrate on SOM, as reflected by increased unlabelled CO2 in soils amended with substrates relative to control soils, were generally lower in shaded treatments than un-shaded treatments. Glucose-amended soils were the exception; PE was greater in the absence of C supply to the rhizosphere. Supporting our second hypothesis, this demonstrates that rhizosphere C supply coupled with the input of substrate amendments accelerates SOM decomposition. In realistic field conditions, different forms of substrates are released into forest soils as litter or rhizodeposits (Kuzyakov 2010). However, our study is among the few that have investigated the combined effect of fresh substrate input and an intact rhizosphere in either forest (Subke et al. 2004) or agro-ecosystems (Mwafulirwa et al. 2017). A potential shortcoming of our simple forest system in this mesocosm study is the absence of the associations of ECM mycelium with more than one tree that may be found in natural forests (Lang et al. 2011), such that altered C supply from one tree host might be compensated by C supply from another tree host. Notwithstanding, our results suggest that in shaded treatments, microorganisms degraded the added substrate to derive energy for microbial growth, which led to co-metabolism of SOM. The PE observed in these shaded treatments was not significantly different among substrates, indicating that the acquired C from decomposition of labile fractions of substrates were not sufficient to sustain microbial activities for long. This suggests that the energy needed to metabolize SOC is greater than the energy acquired from the catabolism of the added substrate (Fontaine et al. 2007).
Soil priming after substrate additions was likely due to microbial activation (Cheng and Kuzyakov 2005). Other studies have also attributed the increase in SOM decomposition to microbial activation in the presence of roots (Zhu et al. 2014; Kumar et al. 2016; Mwafulirwa et al. 2017) or substrates (Blagodatskaya et al. 2011; Blagodatskaya et al. 2014; Shahbaz et al. 2017). Owing to the low N soil content (0.35%), and no addition of fertiliser to the soils before or after planting, the soils were strongly N limited. This N limitation was further aggravated by adding substrates with high C to N ratio at concentrations (3 mg substrate C g−1 soil) high enough to induce SOM decomposition (Luo et al. 2016; Liu et al. 2017). Therefore, it is likely that in a bid to meet their nutrient demands, microorganisms utilized the rhizodeposits or the labile C fraction of substrates as an energy source to decompose the less available more stable SOM, and mobilize nutrients (i.e. the microbial mining hypothesis, (Fontaine et al. 2011)). Similar increased SOM decomposition was observed with the input of substrates with high C:N ratios into subtropical forest soils when compared to nutrient-rich soils (Qiao et al. 2016). This further supports the hypothesis that the direction and magnitude of PE is controlled by the compromise between energy and nutrient availability for SOM decomposition (Fontaine et al. 2003).
In general, the decomposition of complex substrates is dominated by fungi, while bacteria dominate decomposition of soluble substrates (sugars and amino acids) (Fontaine et al. 2011). In the presence of substrates, higher PEs were observed in un-shaded treatments than in shaded treatments with limited C supply to ECM fungi. This suggests that the supply of C by plants to roots and their associated ectomycorrhizae exerts a greater effect on SOM decomposition than the addition of substrates directly to the soil. However, a three-source partitioning of total CO2 efflux is required for further confirmation. Ectomycorrhizal fungi therefore have a significant influence on soil C storage and the magnitude of this influence is dependent on plant productivity (Moore et al. 2015). Under low plant productivity conditions, mycorrhizal fungal biomass is reduced thereby decreasing fungal influence on stored soil C (Moore et al. 2015).
Although both simple and complex substrates increased the decomposition of SOM, we observed higher priming effects in the presence of complex substrates of low microbial availability and higher C:N ratio than from glucose-amended soils. This result does not support our third hypothesis that SOM decomposition is higher following the addition of a readily available substrate (glucose) compared to complex substrates. Glucose is soluble and readily available for microbial utilization, while straw residue, fungal necromass and biochar have complex polymerised C molecules that require more energy for the production of enzymes to decompose them (Luo et al. 2016). Our findings suggest that glucose addition stimulated only microorganisms that are specialised in decomposition of easily utilizable C (r-strategists; Fontaine et al. 2003), while straw, fungal necromass and biochar additions stimulated the activities of microorganisms that are responsible for the decomposition of complex organic molecules by synthesizing extracellular enzymes. Positive PE was therefore due to co-metabolism of SOM during the decomposition of the complex substrates (Kuzyakov et al. 2000; Paterson and Sim 2013).
Biochar has been shown to improve soil fertility as well as reduce soil nutrient losses (Sohi et al. 2010; Quilliam et al. 2012). However, biochar is a recalcitrant material, not readily available for microbial degradation, and the addition of biochar has been reported to have either a negative PE (Cross and Sohi 2011; Cheng et al. 2017), or a positive PE (Wardle et al. 2008; Cui et al. 2017). The feedstock, pyrolysis temperatures and retention duration used during biochar production, together with the age of the biochar and the duration of the experiment can greatly influence potential PE. In a recent study, (Zimmerman and Ouyang 2019) attributed the priming of organic matter by biochar to the presence of habitable surfaces on biochar that encouraged the growth and activities of microbes, hence co-metabolism of SOM. As no mineralization of biochar was observed in this study, positive PE induced by biochar obtained from Miscanthus biomass was likely due to the high stability of biochar that changed the physico-chemical characteristics of soil (e.g. pH, porosity and bulk density), thus promoting SOM decomposition (Sohi et al. 2010; Luo et al. 2017b).
Conclusion
The combination of tree shading and addition of substrates in this forest soil mesocosm experiment enabled us to investigate the effects of rhizosphere C supply and fresh organic matter inputs on the decomposition of SOM. Although the addition of both simple and complex fresh organic matter increased SOM decomposition in shaded treatments, the supply of photoassimilate C to roots and ECM fungi further accelerated SOM decomposition in the presence of fresh organic matter as observed in un-shaded treatments. This suggests that ECM fungi probably increased SOM decomposition through the production of enzymes involved in nutrient mobilization, which was ultimately controlled by the productivity of the host plant. In nutrient-limited and ECM dominated systems such as temperate and boreal forests, interactions between increased rhizodeposition and litter input resulting from higher atmospheric CO2 concentrations (CO2 fertilization effect) may reduce soil C stocks and the sequestration ability of these systems. Better understanding of the mechanisms of PE and the net effect of fresh organic matter input into forest soils might be obtained by measuring extracellular enzyme activities and estimating soil C budgets in forest ecosystems.
Abbreviations
- C:
-
Carbon
- SOM:
-
Soil organic matter
- ECM:
-
Ectomycorrhiza
- N:
-
Nitrogen
- PE:
-
Priming effect
- RPE:
-
Rhizosphere priming effect
References
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545. https://doi.org/10.1038/nature12901
Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A (2006) Root respiration in temperate mountain grasslands differing in land use. Glob Chang Biol 12:995–1006. https://doi.org/10.1111/j.1365-2486.2006.01144.x
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. https://doi.org/10.1007/s00374-008-0334-y
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2007) Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37:95–105. https://doi.org/10.1016/j.apsoil.2007.05.002
Blagodatskaya E, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011) Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol Biochem 43:778–786. https://doi.org/10.1016/j.soilbio.2010.12.011
Blagodatskaya E, Khomyakov N, Myachina O, Bogomolova I, Blagodatsky S, Kuzyakov Y (2014) Microbial interactions affect sources of priming induced by cellulose. Soil Biol Biochem 74:39–49. https://doi.org/10.1016/j.soilbio.2014.02.017
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283. https://doi.org/10.1016/j.soilbio.2010.04.005
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582. https://doi.org/10.1038/nature08930
Brzostek ER, Dragoni D, Brown ZA, Phillips RP (2015) Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol 206:1274–1282. https://doi.org/10.1111/nph.13303
Buée M, Vairelles D, Garbaye J (2005) Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech (Fagus silvatica) forest subjected to two thinning regimes. Mycorrhiza 15:235–245. https://doi.org/10.1007/s00572-004-0313-6
Buée M, Courty PE, Mignot D, Garbaye J (2007) Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal fungal community. Soil Biol Biochem 39:1947–1955. https://doi.org/10.1016/j.soilbio.2007.02.016
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367. https://doi.org/10.1111/gcb.12475
Cheng W, Kuzyakov Y (2005) Root effects on soil organic matter decomposition. In: Agronomy. pp 119–144
Cheng W, Parton WJ, Gonzalez-Meler M a, Phillips R, Asao S, Mcnickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modeling perspectives of rhizosphere priming. New Phytol 201:31–44. https://doi.org/10.1111/nph.12440
Cheng H, Hill PW, Bastami MS, Jones DL (2017) Biochar stimulates the decomposition of simple organic matter and suppresses the decomposition of complex organic matter in a sandy loam soil. GCB Bioenergy 9:1110–1121. https://doi.org/10.1111/gcbb.12402
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Thornton P (2013) Climate change 2013: the physical science basis. Cambridge University Press, New York
Courty PE, Bréda N, Garbaye J (2007) Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol Biochem 39:1655–1663. https://doi.org/10.1016/j.soilbio.2007.01.017
Cross A, Sohi SP (2011) The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol Biochem 43:2127–2134. https://doi.org/10.1016/j.soilbio.2011.06.016
Cui J, Ge T, Kuzyakov Y, Nie M, Fang C, Tang B, Zhou C (2017) Interactions between biochar and litter priming: a three-source 14C and 13C partitioning study. Soil Biol Biochem 104:49–58. https://doi.org/10.1016/j.soilbio.2016.10.014
Di Lonardo DP, De Boer W, Klein Gunnewiek PJA, Hannula SE, Van der Wal A (2017) Priming of soil organic matter: chemical structure of added compounds is more important than the energy content. Soil Biol Biochem 108:41–54. https://doi.org/10.1016/j.soilbio.2017.01.017
Díaz-Pinés E, Schindlbacher A, Pfeffer M, Jandl R, Zechmeister-Boltenstern S, Rubio A (2010) Root trenching: a useful tool to estimate autotrophic soil respiration? A case study in an Austrian mountain forest. Eur J For Res 129:101–109. https://doi.org/10.1007/s10342-008-0250-6
Ekblad A, Högberg P (2000) Analysis of δ13C of CO2 distinguishes between microbial respiration of added C4-sucrose and other soil respiration in a C3-ecosystem. Plant Soil 219:197–209. https://doi.org/10.1023/A:1004732430929
Ekblad A, Wallander H, Godbold DL, Cruz C, Johnson D, Baldrian P, Björk RG, Epron D, Kieliszewska-Rokicka B, Kjøller R, Kraigher H, Matzner E, Neumann J, Plassard C (2013) The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil 366:1–27. https://doi.org/10.1007/s11104-013-1630-3
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21:2082–2094. https://doi.org/10.1111/gcb.12816
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35:837–843. https://doi.org/10.1016/S0038-0717(03)00123-8
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280. https://doi.org/10.1038/nature06275
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. https://doi.org/10.1016/j.soilbio.2010.09.017
Gadgil RL, Gadgil PD (1971) Mycorrhiza and litter decomposition. Nature 233:133–133
Gadgil RL, Gadgil PD (1975) Suppression of litter decomposition by mycorrhizal roots of Pinus radiata. New Zeal J For Sci 5:33–41
Hasibeder R, Fuchslueger L, Richter A, Bahn M (2015) Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol 205:1117–1127. https://doi.org/10.1111/nph.13146
Hasselquist NJ, Metcalfe DB, Inselsbacher E, Stangl Z, Oren R, Näsholm T, Högberg P (2016) Greater carbon allocation to mycorrhizal fungi reduces tree nitrogen uptake in a boreal forest. Ecology 97:1012–1022. https://doi.org/10.1890/15-1222.1
Hobbie E (2006) Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology 87:563–569
Högberg MN, Högberg P (2002) Extramatrical ectomycorrhizal mycelium contributes one-third of microbial biomass and produces, together with associated roots, half the dissolved organic carbon in a forest soil. New Phytol 154:791–795. https://doi.org/10.1046/j.1469-8137.2002.00417.x
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Löfvenius MO, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Huo C, Luo Y, Cheng W (2017) Rhizosphere priming effect: a meta-analysis. Soil Biol Biochem 111:78–84. https://doi.org/10.1016/j.soilbio.2017.04.003
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the k EC value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33. https://doi.org/10.1007/s11104-009-9925-0
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. https://doi.org/10.1016/j.soilbio.2016.08.011
Kumar A, Kuzyakov Y, Pausch J (2016) Maize rhizosphere priming: field estimates using 13C natural abundance. Plant Soil 409:87–97. https://doi.org/10.1007/s11104-016-2958-2
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396. https://doi.org/10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-#
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Friedel J, Stahra K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123:1–22. https://doi.org/10.1016/j.geoderma.2004.01.032
Lang C, Seven J, Polle A (2011) Host preferences and differential contributions of deciduous tree species shape mycorrhizal species richness in a mixed central European forest. Mycorrhiza 21:297–308. https://doi.org/10.1007/s00572-010-0338-y
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620
Lindahl BD, De Boer W, Finlay RD (2010) Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. ISME J 4:872–881. https://doi.org/10.1038/ismej.2010.19
Liu X-JA, Sun J, Mau RL, Finley BK, Compson ZG, van Gestel N, Brown JR, Schwartz E, Dijkstra P, Hungate BA (2017) Labile carbon input determines the direction and magnitude of the priming effect. Appl Soil Ecol 109:7–13. https://doi.org/10.1016/j.apsoil.2016.10.002
Luo Z, Wang E, Sun OJ (2016) A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems. Soil Biol Biochem 101:96–103. https://doi.org/10.1016/j.soilbio.2016.07.011
Luo Y, Lin Q, Durenkamp M, Dungait AJ, Brookes PC (2017a) Soil priming effects following substrates addition to biochar-treated soils after 431 days of pre-incubation. Biol Fertil Soils 53:315–326. https://doi.org/10.1007/s00374-017-1180-6
Luo Y, Zang H, Yu Z, Chen Z, Gunina A, Kuzyakov Y, Xu J, Zhang K, Brookes PC (2017b) Priming effects in biochar enriched soils using a three-source-partitioning approach: 14C labelling and 13C natural abundance. Soil Biol Biochem 106:28–35. https://doi.org/10.1016/j.soilbio.2016.12.006
Marx D (1969) The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59:153–163
Moore JAM, Jiang J, Patterson CM, Mayes MA, Wang G, Classen AT (2015) Interactions among roots, mycorrhizas and free-living microbial communities differentially impact soil carbon processes. J Ecol 103:1442–1453. https://doi.org/10.1111/1365-2745.12484
Moyano FE, Vasilyeva N, Bouckaert L, Cook F, Craine J, Curiel Yuste J, Don A, Epron D, Formanek P, Franzluebbers A, Ilstedt U, Kätterer T, Orchard V, Reichstein M, Rey A, Ruamps L, Subke J-A, Thomsen IK, Chenu C (2012) The moisture response of soil heterotrophic respiration: interaction with soil properties. Biogeosciences 9:1173–1182. https://doi.org/10.5194/bg-9-1173-2012
Mwafulirwa LD, Baggs EM, Russell J, Morley N, Sim A, Paterson E (2017) Combined effects of rhizodeposit C and crop residues on SOM priming, residue mineralization and N supply in soil. Soil Biol Biochem 113:35–44. https://doi.org/10.1016/j.soilbio.2017.05.026
Norby RJ, Delucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, De Angelis P, Finzi AC, Karnosky DF, Kubiske ME, Lukac M, Pregitzer KS, Scarascia-Mugnozza GE, Schlesinger WH, Oren R (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proc Natl Acad Sci U S A 102:18052–18056. https://doi.org/10.1073/pnas.0509478102
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EVJ (2009) Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl Soil Ecol 42:183–190. https://doi.org/10.1016/j.apsoil.2009.03.003
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Glob Chang Biol 19:1562–1571. https://doi.org/10.1111/gcb.12140
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol 24:1–12
Phillips RP, Fahey TJ (2006) Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 87:1302–1313
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179. https://doi.org/10.1007/s004420000578
Phillips L a, Ward V, Jones MD (2014) Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests. ISME J 8:699–713. https://doi.org/10.1038/ismej.2013.195
Qiao N, Xu X, Hu Y, Blagodatskaya E, Liu Y, Schaefer D, Kuzyakov Y (2016) Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Sci Rep 6:19865. https://doi.org/10.1038/srep19865
Quilliam RS, Marsden KA, Gertler C, Rousk J, DeLuca TH, Jones DL (2012) Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agric Ecosyst Environ 158:192–199. https://doi.org/10.1016/j.agee.2012.06.011
Rosling A, Landeweert R, Lindahl BD, Larsson KH, Kuyper TW, Taylor AFS, Finlay RD (2003) Vertical distribution of ectomycorrhizal fungal taxa in a podzol soil profile. New Phytol 159:775–783. https://doi.org/10.1046/j.1469-8137.2003.00829.x
Schlesinger WH, Bernhardt ES (2013) Introduction. In: biogeochemistry, 3rd edn. Elsevier, pp 3–14
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Shahbaz M, Kuzyakov Y, Sanaullah M, Heitkamp F, Zelenev V, Kumar A, Blagodatskaya E (2017) Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils 53:287–301. https://doi.org/10.1007/s00374-016-1174-9
Simpson MJ, Simpson AJ (2012) The chemical ecology of soil organic matter molecular constituents. J Chem Ecol 38:768–784. https://doi.org/10.1007/s10886-012-0122-x
Six J, Paustian K, Elliott ET, Combrink C (2000) Soil structure and organic matter. Soil Sci Soc Am J 64:681. https://doi.org/10.2136/sssaj2000.642681x
Smith SE, Read DJ (2002) Structure and development of ectomycorrhizal roots. In: mycorrhizal Symbiosis, third. Elsevier, p 163–V
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil, 1st edn. Elsevier Inc.
Stewart CE, Zheng J, Botte J, Cotrufo MF (2013) Co-generated fast pyrolysis biochar mitigates green-house gas emissions and increases carbon sequestration in temperate soils. GCB Bioenergy 5:153–164. https://doi.org/10.1111/gcbb.12001
Subke J-A, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559. https://doi.org/10.1007/s00442-004-1540-4
Subke JA, Inglima I, Cotrufo MF (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943. https://doi.org/10.1111/j.1365-2486.2006.01117.x
Subke JA, Voke NR, Leronni V, Garnett MH, Ineson P (2011) Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol 99:186–193. https://doi.org/10.1111/j.1365-2745.2010.01740.x
Talbot JM, Allison SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963. https://doi.org/10.1111/j.1365-2435.2008.01402.x
Tang M, Cheng W, Zeng H, Zhu B (2019) Light intensity controls rhizosphere respiration rate and rhizosphere priming effect of soybean and sunflower. Rhizosphere 9:97–105. https://doi.org/10.1016/J.RHISPH.2018.12.002
Trap J, Akpa-Vinceslas M, Margerie P, Boudsocq S, Richard F, Decaëns T, Aubert M (2017) Slow decomposition of leaf litter from mature Fagus sylvatica trees promotes offspring nitrogen acquisition by interacting with ectomycorrhizal fungi. J Ecol 105:528–539. https://doi.org/10.1111/1365-2745.12665
UK Met Office (2017) No Title. https://www.metoffice.gov.uk/public/weather/climate/gcvpnrf34. Accessed 1 Feb 2017
Vance E, Brookes PC, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Wallander H, Nilsson LO, Hagersberg D, Baath E (2001) Estimation of the biomass and seasonal growth of externalmycelium of ectomycorrhizal fungi in the field. New Phytol 151:753–760
Wallander H, Ekblad A, Godbold DL, Johnson D, Bahr A, Baldrian P, Björk RG, Kieliszewska-Rokicka B, Kjøller R, Kraigher H, Plassard C, Rudawska M (2013) Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils – a review. Soil Biol Biochem 57:1034–1047. https://doi.org/10.1016/j.soilbio.2012.08.027
Wang H, Boutton TW, Xu W, Hu G, Jiang P, Bai E (2015) Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci Rep 5:10102. https://doi.org/10.1038/srep10102
Wardle D a, Nilsson M-C, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science (80- ) 320:629–629. https://doi.org/10.1126/science.1154960
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction: An automated procedure. Soil Biol Biochem 22:1167–1169. https://doi.org/10.1016/0038-0717(90)90046-3
Yin H, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221. https://doi.org/10.1016/j.soilbio.2014.07.022
Yin L, Dijkstra FA, Wang P, Zhu B, Cheng W (2018) Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytol 218:1036–1048
Zak DR, Pregitzer KS, Kubiske ME, Burton AJ (2011) Forest productivity under elevated CO2 and O3: positive feedbacks to soil N cycling sustain decade-long net primary productivity enhancement by CO2. Ecol Lett 14:1220–1226. https://doi.org/10.1111/j.1461-0248.2011.01692.x
Zhang W, Wang X, Wang S (2013) Addition of external organic carbon and native soil organic carbon decomposition: a meta-analysis. PLoS One 8:e54779. https://doi.org/10.1371/journal.pone.0054779
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng W (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192. https://doi.org/10.1016/j.soilbio.2014.04.033
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301. https://doi.org/10.1021/es903140c
Zimmerman AR, Ouyang L (2019) Priming of pyrogenic C (biochar) mineralization by dissolved organic matter and vice versa. Soil Biol Biochem 130:105–112. https://doi.org/10.1016/J.SOILBIO.2018.12.011
Acknowledgments
This project was funded by Petroleum Technology Development Fund (PTDF), Nigeria. The δ13C analyses were funded by a NERC Scientific Facilities and Technology grant (proposal no. CEH_L_095_11_2015).
We would like to thank the NERC life science mass spectrometry facility (LSMSF) for the δ13C analyses. We thank James Weir, Lorna English and Ian Washbourne for technical assistance. We thank Michael Jackson for assistance with fieldwork. We also thank Tom Bradford-Lawrence for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jackson, O., Quilliam, R.S., Stott, A. et al. Rhizosphere carbon supply accelerates soil organic matter decomposition in the presence of fresh organic substrates. Plant Soil 440, 473–490 (2019). https://doi.org/10.1007/s11104-019-04072-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04072-3