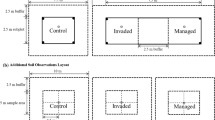
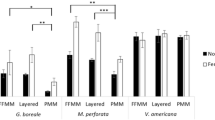
Abstract
Background and aims
Wollemi pine (Wollemia nobilis Jones, Hill & Allen) is a critically endangered conifer and living fossil. Translocation has been proposed as a conservation strategy to establish ‘back-ups’ to the wild population; however, knowledge regarding the environmental and biotic requirements of individuals planted in new environments is limited.
Methods
An experimental translocation was established in a new location in the wild with Wollemi pines planted along a light and elevation gradient. Specific abiotic soil properties and associated microbial communities were linked to Wollemi pine performance in these new locations to inform best practice for future translocations.
Results
Our results indicate that soil properties can be used to select appropriate translocation sites that ensure initial establishment and growth. One year after translocation Wollemi pine had recruited a species-specific fungal community that persisted. Species-specific bacterial communities in their soil and roots formed in the second year after planting. Translocated Wollemi pines that were unhealthy and were not growing did not have the species-specific fungal community.
Conclusion
The long-term functional consequence of this species-specific microbial community warrants ongoing investigation. This is one of the first studies to explicitly consider the role of microbial communities during the translocation of a rare plant and such approaches will be valuable for informing best translocation practice for other rare plant species.





Similar content being viewed by others
References
Abdo Z, Schüette U, Bent S, et al. (2006) Statistical methods for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environ Microbiol 8:929–938. doi:10.1111/j.1462-2920.2005.00959.x
Abeli T, Dixon K (2016) Translocation ecology: the role of ecological sciences in plant translocation. Plant Ecol editorial:1–3. doi:10.1007/s11258-016-0575-z
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. doi:10.1007/s005720100097
Augé R, Toler H, Saxton A (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24. doi:10.1007/s00572-014-0585-4
Bais HP, Weir TL, Perry LG, et al. (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. doi:10.1146/annurev.arplant.57.032905.105159
Bates D, Mächler M, Bolker B, et al. (2015) Fitting Linear Mixed-Effects Models Using. J Stat Softw 67(1)
Benson J, Allen C (2007) Vegetation associated with Wollemia nobilis (Araucariaceae). Cunninghamia 10:255–262
Bever J (1994) Feedback between plants and their soil communities in an old field community. Ecology 75:1965–1977
Bever JD, Platt TG, Morton ER (2012) Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol 66:265
Bieleski R (1959) Factors affecting growth and distribution of kauri (Agathis australis Salisb.) III. Effect of temperature and soil conditions. Aust J Bot 7:279. doi:10.1071/BT9590279
Biggs, L (2009) Mycorrhizal inoculation, endophytic colonization, and allelopathic potential of the Wollemi pine (Wollemia nobilis). Masters Thesis, The University of British Columbia.
Bonanomi G, Giannino F, Mazzoleni S (2005) Negative plant–soil feedback and species coexistence. Oikos 111:311–321. doi:10.1111/j.0030-1299.2005.13975.x
Bray R, Kurtz L (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Chemidlin Prévost-Bouré N, Dequiedt S, Thioulouse J, et al. (2014) Similar processes but different environmental filters for soil bacterial and fungal community composition turnover on a broad spatial scale. PLoS One 9:e111667. doi:10.1371/journal.pone.0111667
Clark D, Clark DB (1984) Spacing dynamics of a tropical rain forest tree: evaluation of the Janzen-Connell model. Am Nat 124:769–788
Connell J (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rainforest trees. In: Boer D, Gradwell G (eds) Dynamics of Populations- Proceedings of the Advanced Study Institute. Centre for Agricultural Publishing and Documentation, Wageningen, pp. 298–312
Culman SW, Bukowski R, Gauch HG, et al (2009) T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinformatics 10:171.
Curlevski NJA, Xu Z, Anderson IC, Cairney JWG (2010) Converting Australian tropical rainforest to native Araucariaceae plantations alters soil fungal communities. Soil Biol Biochem 42:14–20. doi:10.1016/j.soilbio.2009.08.001
R Development Core Team (2013) R: A language and environment for statistical computing. R Found. Stat. Comput
Donaldson JS (2009) Botanic gardens science for conservation and global change. Trends Plant Sci 14:608–613. doi:10.1016/j.tplants.2009.08.008
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annu Rev Environ Resour 30:75–115. doi:10.1146/annurev.energy.30.050504.144212
Eviner VT, Hawkes CV (2008) Embracing variability in the application of plant-soil interactions to the restoration of communities and ecosystems. Restor Ecol 16:713–729. doi:10.1111/j.1526-100X.2008.00482.x
Fox J, Weisberg S (2011) An R Companion to Applied Regression, 2nd edn. Thousand Oaks, California
Gardes M, Bruns T (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
Godefroid S, Piazza C, Rossi G, et al. (2011) How successful are plant species reintroductions? Biol Conserv 144:672–682. doi:10.1016/j.biocon.2010.10.003
Gordon D (1996) Experimental translocation of the endangered shrub Apalachicola rosemary Conradina glabra to the Apalachicola bluffs and ravines preserve, Florida. Biol Conserv 77:19–26
Gosling P, Jones J, Bending GD (2016) Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management. Mycorrhiza 26:77–83. doi:10.1007/s00572-015-0651-6
Guerrant EO, Kaye TN (2007) Reintroduction of rare and endangered plants: common factors, questions and approaches. Aust J Bot 55:362–370
Handreck K, Black N (1999) Growing media for ornamental plants and turfs. University of New South Wales Press, Sydney
Harris J (2009) Soil microbial communities and restoration ecology: facilitators or followers? Science 325(80-):573–574. doi:10.1126/science.1172975
Hauben L, Vauterin L, Swings J, Moore E (1997) Comparison of 16S ribosomal DNA sequence of all Xanthomonas species. Int J Syst Evol Microbiol 47:328–335
Janzen D (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:592–595
Jasper DA (2007) Beneficial soil microorganisms of the Jarrah forest and their recovery in bauxite mine restoration in southwestern Australia. Restor Ecol 15:74–84. doi:10.1111/j.1526-100X.2007.00295.x
Jusaitis M, Polomka L, Sorensen B (2004) Habitat specificity, seed germination and experimental translocation of the endangered herb. Brachycome muelleri (Asteraceae) 116(2):251–266
Kaye TN (2008) Vital steps toward success of endangered plant reintroductions. Nativ Plants J 9:313–322
Klironomos J (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Lankau RA (2013) Species invasion alters local adaptation to soil communities in a native plant. Ecology 94:32–40. doi:10.1890/12-0675.1
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120. doi:10.1128/AEM.00335-09
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Liu X, Ellsworth DS, Tyree MT (1997) Leaf nutrition and photosynthetic performance of sugar maple (Acer saccharum) in stands with contrasting health conditions. Tree Physiol 17:169–178. doi:10.1093/treephys/17.3.169
Mangan SA, Schnitzer SA, Herre EA, et al (2010) Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466:752–755. doi: http://www.nature.com/nature/journal/v466/n7307/abs/nature09273.html#supplementary-information.
Marchesi J, Sato T, Weightman A, et al. (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
McGee P, Bullock S, Summerell B (1999) Structure of mycorrhizae of the Wollemi pine (Wollemia noblis) and related Araucariaceae. Aust J Bot 47:5–95
Menges ES (2008) Turner review no. 16. Restoration demography and genetics of plants: when is a translocation successful? Aust J Bot 56:187–196
Middleton EL, Bever JD (2012) Inoculation with a native soil community advances succession in a grassland restoration. Restor Ecol 20:218–226. doi:10.1111/j.1526-100X.2010.00752.x
Monks L, Coates D, Bell T, Bowles M (2012) Determining success criteria for reintroductions of threatened long-lived plants. In: Plant reintroduction in a changing climate: promises and perils Island Press, Washington DC
Murphy CA, Foster BL (2014) Soil properties and spatial processes influence bacterial Metacommunities within a grassland restoration experiment. Restor Ecol 22:685–691. doi:10.1111/rec.12127
NSW Department of Environment and Conservation (2006) Wollemi Pine (Wollemia nobilis) Recovery Plan. NSW Department of Environment and Conservation, Hurstville, pp. 1–37
Nuñez MA, Horton TR, Simberloff D (2009) Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90:2352–2359
Offord C, Porter C, Meagher P, Errington G (1999) Sexual reproduction and early plant growth of the Wollemi pine (Wollemia nobilis) a rare and threatened Australian conifer. Ann Bot 84:1–9
Offord CA, Meagher PF, Zimmer HC (2014) Growing up or growing out? How soil pH and light affect seedling growth of a relictual rainforest tree. AoB. Plants 6:plu011. doi:10.1093/aobpla/plu011
Oksanen J, Blanchet F, Kindt R, et al (2007) vegan: community ecology package. R Packag. http://www.CRAN.R-project.org/package=vegan
Plassart P, Akpa Vinceslas M, Gangneux C, et al. (2008) Molecular and functional responses of soil microbial communities under grassland restoration. Agric Ecosyst Environ 127:286–293. doi:10.1016/j.agee.2008.04.008
Potthoff M, Steenwerth KL, Jackson LE, et al. (2006) Soil microbial community composition as affected by restoration practices in California grassland. Soil Biol Biochem 38:1851–1860. doi:10.1016/j.soilbio.2005.12.009
Powell JR, Bennett AE (2015) Unpredictable assembly of arbuscular mycorrhizal fungal communities. Pedobiologia (Jena) 59:11–15. doi:10.1016/j.pedobi.2015.12.001
Powell JR, Karunaratne S, Campbell CD, et al. (2015) Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat Commun 6:1–10
Richardson DM, Allsopp N, D’Antonio CM, et al. (2000) Plant invasions — the role of mutualisms. Biol Rev 75:65–93. doi:10.1111/j.1469-185X.1999.tb00041.x
Rigg J, Offord C, BK S, et al. (2016a) Variation in soil microbial communities associated with critically endangered Wollemi pine affects fungal, but not bacterial, assembly within seedling roots. Pedobiologia (Jena) 59:61–71. doi:10.1016/j.pedobi.2016.02.002
Rigg J, (2016b) Microbial influences on the conservation and recovery of wollemi pine. PhD Thesis. Western Sydney University (in press)
Rillig MC, Antonovics J, Caruso T, et al. (2015) Interchange of entire communities: microbial community coalescence. Trends Ecol Evol 30:470–476. doi:10.1016/j.tree.2015.06.004
Rillig MC, Lehmann A, Aguilar-Trigueros C, et al. (2016) Soil microbes and community coalescence. Pedobiologia (Jena) 59:37–40. doi:10.1016/j.pedobi.2016.01.001
Rousk J, Bååth E, Brookes PC, et al. (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. doi:10.1038/ismej.2010.58
Seal Analytic (2011) Method Number: EPA-118_A Rev. 5: o-Phosphate-P in drinking, saline and surface waters, and domestic and industrial wastes. http://www.seal-analytical.com/Methods/DiscreteMethods/AQ2EPAMethods/tabid/76/language/en-US/Default.aspx.
Silvester WB, Orchard TA (1999) The biology of kauri ( Agathis australis ) in New Zealand. Production, biomass, carbon storage, and litter fall in four forest remnants. New zeal. J Bot 37:553–571. doi:10.1080/0028825X.1999.9512653
Singh B, Nazaries L, Munro S, et al. (2006) Use of multiplex terminal restriction fragment length polymorphism for rapid and simultaneous analysis of different components of the soil microbial community. Appl Environ Microbiol 72:7278–7285
Smith SE, Read DJ (1997) Chapter 17: Mycorrhizas in managed environments: Forest production, interactions with other microorganisms and pollutants. In: Mycorrhizal Symbiosis (Second Edition). Academic Press, London, pp 470–489
Smith C, Danilowicz B, Clear A, et al. (2005) T-align, a web-based tool for comparison of multiple terminal restriction fragment length polymorphism profiles. FEMS Microbiol Ecol 54:375–380. doi:10.1016/j.femsec.2005.05.002
Tedersoo L, Bahram M, Polme S, et al. (2014) Global diversity and geography of soil fungi. Science 346(80-):1256688–1256688. doi:10.1126/science.1256688
Thomas P (2011) The IUCN Red List of Threatened Species 2011: e.T34926 A9898196. www.iucnredlist.org. Accessed 29 Mar 2016
Vallee L, Hogbin T, Monks L, et al. (2004) Guidelines for the translocation of threatened plants in Australia, 2nd Editio. Australian Network for Plant Conservation, Canberra
Walder F, van der Heijden MGA (2015) Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nat Plants 1:15159. doi:10.1038/nplants.2015.159
White B, Taylor DL (1990) Analysis of phylogenetic relationship by amplification and direct sequencing of ribosomal RNA genes. In: PCR protocol: a guide to method and applications Academic Press, New York, pp 315–322
Wyse S (2012) Growth responses of five forest plant species to the soils formed beneath New Zealand kauri (Agathis australis). New zeal. J Bot 50:411–421. doi:10.1080/0028825X.2012.724428
Yang C-H, Crowley DE (2000) Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl Environ Microbiol 66:345–351. doi:10.1128/AEM.66.1.345-351.2000
Zimmer HC, Offord CA, Auld TD, Baker PJ (2016) Establishing a wild, ex situ population of a critically endangered shade-tolerant rainforest conifer: a translocation experiment. PLoS One 11:e0157559. doi:10.1371/journal.pone.0157559
Zoë FS, Elizabeth AJ, Mark JM, Cassandra BM (2009) Planting conditions improve translocation success of the endangered terrestrial orchid Diuris fragrantissima (Orchidaceae. Aust J Bot 57:200–209. doi:10.1071/BT09072
Acknowledgments
We would like to thank Tony Auld and the Wollemi pine recovery team for their approval to conduct the experimental translocation and approval to use plants from the ex situ collection of Wollemi pine. We gratefully acknowledge Dave Crestani (The Blue Mountains Botanic Gardens, Mount Tomah) for identifying plant species during the vegetation survey and also The Blue Mountains Botanic Gardens team (Mount Tomah) for assistance planting Wollemi pine and help with ongoing management of the site. Jessica Rigg was supported by an Australian Postgraduate Award from the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Duncan D. Cameron.
Electronic supplementary material
Supplementary Table 1
(DOCX 13 kb)
Supplementary Table 2
(DOCX 16 kb)
Supplementary Figure 1
(DOCX 1011 kb)
Supplementary Figure 2
(DOCX 40 kb)
Supplementary Figure 3
Total water holding capacity (TWHC) and air-filled porosity (AFP) of soil at 768 the time Wollemi pine was planted. There was no significant difference between TWHC or AFP 769 according to gap type. (PDF 4 kb)
Rights and permissions
About this article
Cite this article
Rigg, J.L., Offord, C.A., Zimmer, H. et al. Conservation by translocation: establishment of Wollemi pine and associated microbial communities in novel environments. Plant Soil 411, 209–225 (2017). https://doi.org/10.1007/s11104-016-3010-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-3010-2