Abstract
Aims
Rytidosperma species are native Australian grasses which have different growth rates and phosphorus (P) requirements. This study examined the role of root morphology traits in response to P supply.
Methods
Nine Rytidosperma species ranging from slow- to fast-growth were examined along with Lolium perenne and Bromus hordeaceus. Plants were grown in a glasshouse for 47 days in soil supplied with six levels of P between 0 and 60 mg P per pot. Root mass, length and diameter, root hair length and density, and extent of mycorrhizal colonisation were measured.
Results
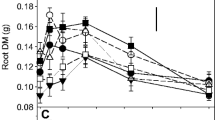
Across all species there was a positive correlation (P < 0.001) between P uptake and root mass, length and root hair cylinder volume (RHCV; estimated using root diameter, root hair length and root length) at all levels of P supply. An exception was the RHCV of B. hordeaceus, where expected P uptake was not achieved due to a markedly reduced root length at low-P supply. For the Rytidosperma species, morphological plasticity for specific root length, root mass fraction and root hair length ranged from 1.5-fold to 2.7-fold between high- and low-P supply. However, across all species and P levels no single root morphological trait was identified for universally increasing the size of the root system and P uptake.
Conclusions
Fast-growing species took up more P as a result of an overall larger root mass, greater root length and larger RHCV.





Similar content being viewed by others
References
Abbott LK, Robson A (1977) Growth stimulation of subterranean clover with vesicular arbuscular mycorrhizas. Aust J Agric Res 28:639–649
Barrett DJ, Gifford RM (1999) Increased C-gain by an endemic Australian pasture grass at elevated atmospheric CO2 concentration when supplied with non-labile inorganic phosphorus. Funct Plant Biol 26:443–451
Bates TR, Lynch JP (2000) The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am J Bot 87:964–970
Bouma TJ, Nielsen KL, Koutstaal B (2000) Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant Soil 218:185–196
Brown LK, George TS, Dupuy LX, White PJ (2013) A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Ann Bot 112:317–330
Burkitt L, Moody P, Gourley C, Hannah M (2002) A simple phosphorus buffering index for Australian soils. Soil Res 40:497–513
Chapin FS (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Christie EK, Moorby J (1975) Physiological responses of semi-arid grasses. I. The influence of phosphorus supply on growth and phosphorus absorption. Aust J Agric Res 26:423–436
Colwell J (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern new South Wales by soil analysis. Aust J Exp Agric Anim Husb 3:190–197
Crush J, Ouyang L, Nichols S (2010) Loss of weight in ryegrass and clover roots preserved in ethanol prior to image analysis for root traits. Acta Physiol Plant 32:605–606
Donald CM (1970) Temperate pasture species. In: Moore RM (ed) Australian grasslands. Australia, Australian National University Press, Canberra, pp. 303–320
Drew M (1975) Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75:479–490
Eissenstat DM (1991) On the relationship between specific root length and the rate of root proliferation: a field study using citrus rootstocks. New Phytol 118:63–68
Eissenstat DM (1992) Costs and benefits of constructing roots of small diameter. J Plant Nutr 15:763–782
Flavel RJ, Guppy CN, Tighe MK, Watt M, Young IM (2014) Quantifying the response of wheat (Triticum aestivum L) root system architecture to phosphorus in an Oxisol. Plant Soil 385:303–310
Fohse D, Claassen N, Jungk A (1991) Phosphorus efficiency of plants II. Significance of root radius, root hairs and cation-anion balance for phosphorus influx in seven plant species. Plant Soil 132:261–272
Gahoonia TS, Care D, Nielsen NE (1997) Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 191:181–188
Garden DL, Dowling PM, Eddy DA, Nicol HI (2001) The influence of climate, soil, and management on the composition of native grass pastures on the central, southern, and Monaro tablelands of new South Wales. Aust J Agric Res 52:925–936
Garden DL, Ellis NJS, Rab MA, Langford CM, Johnston WH, Shields C, Murphy T, Holmberg M, Dassanayake KB, Harden S (2003) Fertiliser and grazing effects on production and botanical composition of native grasslands in south-east Australia. Aust J Exp Agric 43:843–859
Garden DL, Lodge GM, Friend DA, Dowling PM, Orchard BA (2000) Effects of grazing management on botanical composition of native grass-based pastures in temperate south-East Australia. Aust J Exp Agric 40:225–245
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Grace C, Stribley DP (1991) A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol Res 95:1160–1162
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester, UK
Haling RE, Richardson AE, Culvenor RA, Lambers H, Simpson RJ (2010) Root morphology, root-hair development and rhizosheath formation on perennial grass seedlings is influenced by soil acidity. Plant Soil 335:457–468
Handreck KA (1997) Phosphorus requirements of Australian native plants. Aust J Soil Res 35:241–289
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Hodge A, Robinson D, Griffiths B, Fitter A (1999) Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ 22:811–820
Isbell RF (2002) The Australian soil classification. Melbourne, Vic, CSIRO Publishing
Jakobsen I, Chen BD, Munkvold L, Lundsgaard T, Zhu YG (2005) Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant Cell Environ 28:928–938
Jungk A, Barber S (1974) Phosphate uptake rate of corn roots as related to the proportion of the roots exposed to phosphate. Agron J 66:554–557
Koide RT, Mosse B (2004) A history of research on arbuscular mycorrhiza. Mycorrhiza 14:145–163
Lambers H, Poorter H (1992) Inherent variation in growth-rate between higher-plants - a search for physiological causes and ecological consequences. Adv Ecol Res 23:187–261
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst 108:361–367
Nielsen KL, Eshel A, Lynch JP (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 52:329–339
O’Dwyer C, Attiwill PM (1999) A comparative study of habitats of the golden sun moth Synemon plana Walker (Lepidoptera: Castniidae): implications for restoration. Biol Conserv 89:131–141
Otani T, Ae N (1996) Sensitivity of phosphorus uptake to changes in root length and soil volume. Agron J 88:371–375
Robinson D, Van Vuuren MMI (1998) Responses of wild plants to nutrient patches in relation to growth rate and life-form. In: Lambers H, Poorter H, Van Vuuren MMI (eds) Inherent variation in plant growth. Backhuys Publishers, Physiological mechanisms and ecological consequences. Leiden, pp. 237–257
Ryser P (1998) Intra- and interspecific variation in root length, root turnover and the underlying parameters. In: Lambers H, Poorter H, Van Vuuren MMI (eds) Inherent variation in plant growth. The Netherlands, Backhuys Publishers, Physiological mechanisms and ecological consequences. Leiden, pp. 441–465
Snapp SS, Lynch JP (1996) Phosphorus distribution and remobilization in bean plants as influenced by phosphorus nutrition. Crop Sci 36:929–935
Snaydon R, Howe C (1986) Root and shoot competition between established ryegrass and invading grass seedlings. J Appl Ecol 23:667–674
Stephens CG, Donald CM (1959) Australian soils and their responses to fertilisers. Adv Agron 10:167–256
Van de Vijver C, Boot R, Poorter H, Lambers H (1993) Phenotypic plasticity in response to nitrate supply of an inherently fast-growing species from a fertile habitat and an inherently slow-growing species from an infertile habitat. Oecologia 96:548–554
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Vierheilig H, Schweiger P, Brundrett M (2005) An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol Plant 125:393–404
Waddell HA, Simpson RJ, Henderson B, Ryan MH, Lambers H, Garden DL, Richardson AE (2015) Differential growth response of Rytidosperma species (wallaby grass) to phosphorus application and implications for grassland management. Grass Forage Sci 71:245–258
Waddell HA, Simpson RJ, Lambers H, Henderson B, Ryan MH, Garden DL, Richardson AE (2016) Phosphorus utilisation efficiency and leaf morphology traits of Rytidosperma (wallaby grass) that differ in their growth response to phosphorus fertilisation. Aust J Bot 64:65–76
Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148:459–471
Waters CM, Melville G, Jacobs S (2009) Association of five Austrodanthonia species (family Poaceae) with large and small scale environmental features in central western New South Wales. Cunninghamia 11:65–80
White RE (1972) Studies on mineral ion absorption by plants I. The absorption and utilisation of phosphate by Stylonsanthes humili, Phaseolus atropurpureus, and Desmodium intortum. Plant Soil 36:427–447
Wissuwa M, Ae N (2001) Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed 120:43–48
Wissuwa M, Gamat G, Ismail AM (2005) Is root growth under phosphorus deficiency affected by source or sink limitations? J Exp Bot 56:1943–1950
Acknowledgments
This research was funding by an Australian Postgraduate Award and a Grains Research and Development Corporation Industry Research Scholarship to HAW. We thank Adam Stefanski, Zongjian Yang and Branka Culvenor for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim S. George.
Rights and permissions
About this article
Cite this article
Waddell, H.A., Simpson, R.J., Ryan, M.H. et al. Root morphology and its contribution to a large root system for phosphorus uptake by Rytidosperma species (wallaby grass). Plant Soil 412, 7–19 (2017). https://doi.org/10.1007/s11104-016-2933-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2933-y