Abstract
Background and aims
The impact of salinity on microbes has been studied extensively but little is known about the response of soil microbial activity and biomass to increasing salinity in rhizosphere compared to bulk (non-rhizosphere) soil.
Methods
Barley was grown for 5 weeks in non-saline loamy sand to which salt (NaCl) was added. The electrical conductivity in the saturated extract (ECe) was 1, 13 and 19 dS m−1 for non-saline and two saline soils. Pots without plants were prepared in the same manner and placed next to those with plants. The water content in all pots was maintained at 75 % of water-holding capacity by weight. After 5 weeks the planted and unplanted pots were harvested to collect rhizosphere and bulk soil, respectively. The collected soil was then used for an incubation experiment. The EC levels in the pot experiment (EC1, EC13 and EC19, referred to as original) were either maintained or increased by adding NaCl to adjust the EC to 13, 19, 31 and 44 dS m−1. CO2 release was measured continuously for 20 days, microbial biomass C (MBC) was measured at the start and the end of the incubation experiment.
Results
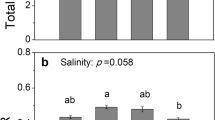
In general, cumulative respiration and microbial biomass C concentration in rhizosphere and bulk soil decreased to a similar extent with increasing adjusted EC. However, compared to the treatments where the EC was maintained, the percentage decrease in cumulative respiration when the EC was increased to EC44 was smaller in rhizosphere than in bulk soil.
Conclusion
Overall, the reduction of cumulative respiration with increasing salinity did not differ between rhizophere and bulk soil. But microbes in rhizosphere soil were more tolerant to high EC than those in bulk soil which could be due to the greater substrate availability in the rhizosphere even after the soil was removed from the roots.



Similar content being viewed by others
References
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods. CAB International
Andronov E, Petrova S, Pinaev A, Pershina E, Rakhimgalieva SZ, Akhmedenov K, Gorobets A, Sergaliev NK (2012) Analysis of the structure of microbial community in soils with different degrees of salinization using T-RFLP and real-time PCR techniques. Eurasian Soil Sci 45(2):147–156
Asghar HN, Setia R, Marschner P (2012) Community composition and activity of microbes from saline soils and non-saline soils respond similarly to changes in salinity. Soil Biol Biochem 47:175–178
Baumann K, Marschner P (2013) Effects of salinity on microbial tolerance to drying and rewetting. Biogeochemistry 112:71–80
Blagodatskaya E, Blagodatsky S, Anderson T-H, Kuzyakov Y (2007) Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37(1):95–105
Chowdhury N, Marschner P, Burns R (2011) Response of microbial activity and community structure to decreasing soil osmotic and matric potential. Plant Soil 344(1):241–254
De Nobili M, Contin M, Mondini C, Brookes P (2001) Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33(9):1163–1170
Demoling LA, Bååth E (2008) Use of pollution–induced community tolerance of the bacterial community to detect phenol toxicity in soil. Environ Toxicol Chem 27:334–340
Demoling F, Figueroa D, Bååth E (2007) Comparison of factors limiting bacterial growth in different soils. Soil Biol Biochem 39(10):2485–2495
Dennis PG, Miller AJ, Hirsch PR (2010) Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol Ecol 72(3):313–327
Dijkstra FA, Cheng W, Johnson DW (2006) Plant biomass influences rhizosphere priming effects on soil organic matter decomposition in two differently managed soils. Soil Biol Biochem 38(9):2519–2526
Elmajdoub B, Marschner P (2013) Salinity reduces the ability of soil microbes to utilise cellulose. Biol Fertil Soils 49(4):379–386
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35(6):837–843
Fu S, Cheng W (2002) Rhizosphere priming effects on the decomposition of soil organic matter in C4 and C3 grassland soils. Plant Soil 238(2):289–294
Hagemann M (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35(1):87–123
Khan KS, Gattinger A, Buegger F, Schloter M, Joergensen RG (2008) Microbial use of organic amendments in saline soils monitored by changes in the 13C/12C ratio. Soil Biol Biochem 40(5):1217–1224
Kuzyakov Y (2002) Review: Factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165(4):382–396
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic, London
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nelson DR, Mele PM (2007) Subtle changes in rhizosphere microbial community structure in response to increased boron and sodium chloride concentrations. Soil Biol Biochem 39(1):340–351
Oren A (2001) The bioenergetic basis for the decrease in metabolic diversity at increasing salt concentrations: implications for the functioning of salt lake ecosystems. Hydrobiologia 466(1):61–72
Oren A, Heldal M, Norland S, Galinski EA (2002) Intracellular ion and organic solute concentrations of the extremely halophilic bacterium Salinibacter ruber. Extremophiles 6(6):491–498
Rayment G, Higginson F (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press Pty Ltd, Sydney
Rengasamy P (2006) Soil salinity and sodicity. In: Stevens D (ed) Growing crops with reclaimed wastewater. CSIRO Publishing
Rengasamy P, Greene R, Ford G, Mehanni A (1984) Identification of dispersive behaviour and the management of red-brown earths. Soil Res 22:413–431
Rietz DN, Haynes RJ (2003) Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol Biochem 35(6):845–854
Rousk J, Elyaagubi FK, Jones DL, Godbold DL (2011) Bacterial salt tolerance is unrelated to soil salinity across an arid agroecosystem salinity gradient. Soil Biol Biochem 43(9):1881–1887
Setia R, Marschner P (2012) Carbon mineralization in saline soils as affected by residue composition and water potential. Biol Fertil Soils 49:71–77
Setia R, Smith P, Marschner P, Baldock J, Chittleborough DJ, Smith J (2011a) Introducing a decomposition rate modifier in the Rothamsted carbon model to predict soil organic carbon stocks in saline soils. Environ Sci Technol 45:6396–6403
Setia R, Marschner P, Baldock J, Chittleborough D, Smith P, Smith J (2011b) Salinity effects on carbon mineralization in soils of varying texture. Biol Biochem 43(9):1908–1916
Tripathi S, Kumari S, Chakraborty A, Gupta A, Chakrabarti K, Bandyapadhyay BK (2006) Microbial biomass and its activities in salt-affected coastal soils. Biol Fertil Soils 42(3):273–277
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707
Wichern J, Wichern F, Joergensen RG (2006) Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 137(1-2):100–108
Wong V, Dalal R, Greene R (2008) Salinity and sodicity effects on respiration and microbial biomass of soil. Biol Fertil Soils 44:943–953
Yan N, Marschner P (2012) Response of microbial activity and biomass to increasing salinity depends on the final salinity, not the original salinity. Soil Biol Biochem 53:50–55
Yan N, Marschner P (2013) Response of soil respiration and microbial biomass to changing EC in saline soils. Soil Biol Biochem 65:322–328
Yuan B-C, Li Z-Z, Liu H, Gao M, Zhang Y-Y (2007) Microbial biomass and activity in salt affected soils under arid conditions. Appl Soil Ecol 35(2):319–328
Acknowledgments
The senior author thanks the Libyan government for the postgraduate scholarship. We thank the anonymous reviewer for the constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Eric Paterson.
Rights and permissions
About this article
Cite this article
Elmajdoub, B., Barnett, S. & Marschner, P. Response of microbial activity and biomass in rhizosphere and bulk soils to increasing salinity. Plant Soil 381, 297–306 (2014). https://doi.org/10.1007/s11104-014-2127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2127-4