Abstract
Aims
The efficacy of N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid (HBED) as an Fe source in plant nutrition for soybean (Glycine max) plants grown in calcareous soil under controlled conditions was studied.
Methods
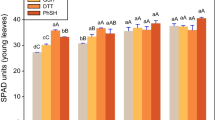
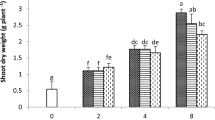
The ability of ethylenediamine-N,N′-bis(o-hydroxyphenylacetic) acid (o,oEDDHA/57Fe3+) and HBED/57Fe3+ at increasing concentrations and the long lasting effect were evaluated. In addition, iron nutrition was studied considering the Fe uptake from the chelates prepared with the isotope 57Fe. Further, the SPAD index, total Fe and 57Fe content in plant were analyzed and soluble and available 57Fe fractions in soil were determined over time.
Results
Doses experiment indicated that a higher concentration of HBED/57Fe3+ as compared to o,oEDDHA/57Fe3+ is necessary for obtaining the same 57Fe absorption by the plant; however, these differences were found to be lower in the second sampling time as compared to the first one. This long lasting effect of HBED/Fe3+ was corroborated in the long term experiment. Moreover, it was found that the load of Fe in the pods was higher when using HBED/57Fe3+ than when o,oEDDHA/57Fe3+ was used. The soil extract analysis for each sampling time indicated that HBED/57Fe3+ presented a higher stability in soil than o,oEDDHA/57Fe3+ over time.
Conclusion
The HBED/Fe3+ could be a long lasting alternative to EDDHA/Fe3+ for correcting the iron chlorosis of dicotyledonous plants grown on calcareous soils.





Similar content being viewed by others
Abbreviations
- HBED:
-
N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid
- o,oEDDHA:
-
Ethylenediamine-N,N′-bis(o-hydroxyphenylacetic) acid
- o,pEDDHA:
-
Ethylenediamine-N-(o-hydroxyphenylacetic acid)-N′-(p-hydroxyphenylacetic acid)
- p,pEDDHA:
-
Ethylenediamine-N,N′-(p-hydroxyphenylacetic) acid
- EDDHA/Fe3+ :
-
Commercial products based on ferric ethylenediamine-N,N′-bis(o-hydroxyphenylacetate) isomer
- DTPA:
-
Diethylenetriaminepentaacetic acid
- AAS:
-
Atomic absorption spectroscopy
- HPLC:
-
High-performance liquid chromatography
- ICP-MS:
-
Inductively coupled plasma mass spectroscopy
- DAT:
-
Days after treatment
- NS:
-
Nutrient solution
References
Abadía J, Vázquez S, Rellán-Álvarez R, El-Jendoubi H, Abadía A, Álvarez-Fernández A, López-Millán AF (2011) Towards a knowledge-based correction of iron chlorosis. Plant Physiol Biochem 49:471–482
Álvarez-Fernández A, Pérez-Sanz A, Lucena JJ (2001) Evaluation of effect of washing procedures on minerals analysis of orange and peach leaves sprayed with seaweed extracts enriched with iron. Commun Soil Sci Plant Anal 32:157–170
Álvarez-Fernández A, Sierra MA, Lucena JJ (2002) Reactivity of synthetic Fe chelates with soils and soil components. Plant Soil 241:129–137
Barak P, Chen Y (1987) Determination of FeEDDHA in soils and fertilizers by anion exchange chromatography. Soil Sci Soc Am J 51:893–896
Bergeron RJ, Wiegand J, Brittenham GM (2002) HBED ligand: preclinical studies of a potential alternative to deferoxamine for treatment of chronic iron overload and acute iron poison. Blood 99:3019–3026
Brittenham GM (1992) Development of iron-chelating agents for clinical use. Blood 80:569–574
CEN (Comité Européen de Normalisation) EN 13368-2 (2011) Fertilizers. Determination of chelating agents in fertilizers by chromatography. Part 2. Determination of Fe chelated by o,o-EDDHA, o,o-EDDHMA and HBED by ion pair chromatography
Chaney RL (1984) Diagnostic practices to identify iron deficiency in higher plants. J Plant Nutr 7:47–67
Chaney RL (1988) Plants can utilize iron from Fe-N, N′-di-(2-hydroxybenzoyl)-ethylene-N, N′-diacetic acid, a ferric chelate with 106 greater formation constant than Fe-EDDHA. J Plant Nutr 11:1033–1050
Escudero R, Gómez-Gallego M, Romano S, Fernández I, Gutiérrez-Alonso A, Sierra MA, López-Rayo S, Nadal P, Lucena JJ (2012) Biological Activity of Fe(III) acquo-complexes towards Ferric Chelate Reductase (FCR). Org Biomol Chem 10:2272–2281
García-Marco S, Martínez N, Yunta F, Hernández-Apaolaza L, Lucena JJ (2006) Effectiveness of ethylenediamine-N-(o-hydroxyphenylacetic)-N′-(p-hydroxyphenylacetic) acid (o, p-EDDHA) to supply iron to plants. Plant Soil 279:31–40
Gómez-Gallego M, Pellico D, Ramírez-López P, Mancheño MJ, Romano S, de la Torre MC, Sierra MA (2005) Understanding of the mode of action of FeIII-EDDHA as iron chlorosis corrector based on its photochemical and redox behavior. Chem Eur J 11:1–10
Hernández-Apaolaza L, García Marco S, Nadal P, Lucena JJ, Sierra MA, Gómez-Gallego M, Ramírez-López P, Escudero R (2006) Structure and fertilizer properties of byproducts formed in the synthesis of EDDHA. J Agric Food Chem 54:4355–4363
Hill-Cottingham DG (1955) Photosensitivity of iron chelates. Nature 175:347–348
Hill-Cottingham DG, Lloyd-Jones CP (1958) Behaviour of iron chelates in calcareous soils. II. Laboratory experiments with some further chelating agents. Plant Soil 9:189–201
ISO 3696 (1987) Water for analytical laboratory use. Specification and test methods
Jones B Jr (2001) Laboratory guide for conducting soil tests and plant analysis. CRC, Florida, p 384
López-Rayo S, Hernández D, Lucena JJ (2009) Chemical evaluation of HBED/Fe3+ and the novel HJB/Fe3+ chelates as fertilizers to alleviate iron chlorosis. J Agric Food Chem 5:8504–8513
Lucena JJ (2000) Effect of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. J Plant Nutr 23:1591–1606
Lucena JJ (2003) Fe chelates for remediation of Fe chlorosis in strategy I plants. J Plant Nutr 26:1969–1984
Lucena JJ, Chaney R (2007) Response of cucumber plants to low doses of different synthetic iron chelates in hydroponics. J Plant Nutr 30:795–809
Lucena JJ, Manzanares M, Gárate A (1992) Comparative study of the efficacy of commercial Fe-chelates using a new test. J Plant Nutr 15:1995–2006
Lucena JJ, Barak P, Hernández-Apaolaza L (1996) Isocratic ion-pair high-performance liquid chromatographic method for the determination of various iron(III) chelates. J Chromatogr A 727:253–264
Ma R, Motekaitis R, Martell A (1994) Stability of metal ion complexes of N, N′-bis(2 hydroxybenzyl)ethylenediamine-N, N′-diacetic acid. Inorg Chim Acta 224:151–155
Nadal P, Hernández-Apaolaza L, Lucena JJ (2009) Effectiveness of N, N′-bis(2-hydroxy-5-methylbenzyl) ethylenediamine-N, N′-diacetic acid (HJB) to supply iron to dicot plants. Plant Soil 325:65–77
Nawrocki A, Stefaniak F, Mrozek-Niecko A, Olszewski R, (Przedsiebiorstwo Produkcyjno-Consultingowe Adob) (2009) Preparation of N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid and its derivatives. PCT Int. Appl. WO 2009037235 A1 20090326, 2009, p 30
Orera I, Rodríguez-Castrillón J, Moldovan M, García-Alonso J, Abadía A, Abadía J, Álvarez-Fernández A (2010) Using a dual-stable isotope tracer method to study the uptake, xylem transport and distribution of Fe and its chelating agent from stereoisomers of an Fe (III)-chelate used as fertilizer in Fe-deficient strategy I plants. Metallomics 2:646–657
Rodríguez-Castrillón JA, Moldovan M, García Alonso JI, Lucena JJ, García-Tomé ML, Hernández-Apaolaza L (2008) Isotope pattern deconvolution as a tool to study iron metabolism in plants. Anal Bioanal Chem 390:579–590
Rojas CL, Romera FJ, Alcántara E, Pérez-Vicente R, Sariego C, García-Alonso JI, Boned J, Martí G (2008) Efficacy of Fe(o, oEDDHA) and Fe(o, pEDDHA) isomers in supplying Fe to strategy I plants differs in nutrient solution and calcareous soil. J Agric Food Chem 56:10774–10778
Schenkeveld WDC, Reichwein AM, Temminghoff EJM, Riemsdijk WHV (2007) The behaviour of EDDHA isomers in soils as influenced by soil properties. Plant Soil 290:85–102
Schenkeveld WDC, Dijcker R, Reichwein AM, Temminghoff EJM, Riemsdijk WHV (2008) The effectiveness of soil-applied FeEDDHA treatments in preventing iron chlorosis in soybean as a function of the o, o-FeEDDHA content. Plant Soil 303:161–176
Schenkeveld WDC, Temminghoff EJM, Reichwein AM Riemsdijk WHV (2010) FeEDDHA-facilitated Fe uptake in relation to the behavior of FeEDDHA components in the soil-plant system as a function of time and dosage. Plant Soil 332
Soltanpour PN, Schwab AP (1977) A new soil test for simultaneous extraction of macro- and micro-nutrients in alkaline soils. Commun Soil Sci Plant Anal 8:195–207
Wallace A, Muerller R, Lunt OR, Ashcroft RT, Shannon LM (1955) Comparisons of five chelating agents in soils, in nutrient solutions, and in plant responses. Soil Sci 80:101–108
Ware GO, Ohki K, Moon LC (1982) The Mitscherlich plant growth model for determining critical nutrient deficiency levels. Agron J 74:88–91
Yunta F, García-Marco S, Lucena JJ, Gómez-Gallego M, Alcazar R, Sierra MA (2003) Chelating agents related to ethylenediamine bis(2-hydroxyphenyl)acetic acid (EDDHA): synthesis, characterization, and equilibrium studies of the free ligands and their Mg2+, Ca2+, Cu2+, and Fe3+ chelates. Inorg Chem 42:5412–5421
Acknowledgments
This research was carried out with the financial support of PPC ADOB (Poland) and the project AGL2010-18048 of the Spanish Ministry of Education and Science. P. Nadal was on a Spanish Ministry of Science and Education “FPI” pre-doctoral grant co-financed by the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael A. Grusak.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 417 kb)
Rights and permissions
About this article
Cite this article
Nadal, P., García-Delgado, C., Hernández, D. et al. Evaluation of Fe-N,N′-Bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetate (HBED/Fe3+) as Fe carrier for soybean (Glycine max) plants grown in calcareous soil. Plant Soil 360, 349–362 (2012). https://doi.org/10.1007/s11104-012-1246-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1246-z