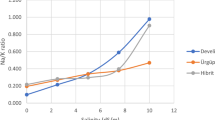
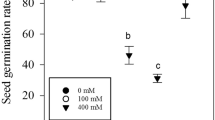
Abstract
Salinity influences plant growth, seed yield and seed quality even of halophytic crops such as Chenopodium quinoa. Plant growth, total seed yield, number of seeds, fresh weight and dry weight of seeds, were all significantly reduced in the presence of salinity. Only at high salinity did the content of proteins (as well as total N) increase significantly in the seeds whereas the content of total carbohydrates (as well as total C) decrease. Aside from that the capacity for germination was diminished by a reduced seed size and a disproportionate reduction of the volume of the perisperm. However, the reduced capacity seemed to be compensated by an accelerated germination owing to high Na and Cl concentrations leading to a low water potential in the walls of the plant ovary. At high salinity the passage of NaCl to the seed interior was hindered by the seed cover. There was an obvious gradient between potentially toxic (Na and Cl) and essentially needed elements (K, Mg, Ca, P and S) across the seed coat of salt treated plants and also a significant change of the distribution of elements in the embryo. The results indicate a highly protected seed interior leading to a high salinity resistance of quinoa seeds.







Similar content being viewed by others
References
Abdullah Z, Khan MA, Flowers TJ (2001) Causes of sterility in seed set of rice under salinity stress. J Agron Crop Sci 187:25–32
Ando H, Chen YC, Tang HJ, Shimizu M, Watanabe K, Mitsunaga T (2002) Food components in fractions of quinoa seed. Food Sci Technol Res 8:80–84
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Boscaiu M, Estrelles M, Soriano P, Vicente O (2005) Effects of salt stress on the reproductive biology of the halophyte Plantago crassifolia. Biol Plant 49:141–143
Bradford MM (1976) Rapid and sensitive method for the quantification of microgram of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brinegar C, Goundan S (1993) Isolation and characterization of chenopodin the 11 S seed storage protein of quinoa (Chenopodium quinoa). J Agric Food Chem 4:182–185
Cano EA, Bolarin MC, Perez-Alfocea F, Caro M (1991) Effect of NaCl priming on increased salt tolerance in tomato. J Hortic Sci 66:621–628
Carden DE, Walker DJ, Flowers TJ, Miller AJ (2003) Single-cell measurements of the contributions of cytosolic Na and K to salt tolerance. Plant Physiol 131:676–683
Castillo RO (1995) Plant genetic resource in the Andes: impact, conservation, and management. Crop Sci 35:355–360
Cayuela E, Perez-Alfocea F, Caro M, Bolarin MC (1996) Priming seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol Plant 96:231–236
Choukr-Allah R (1996) The potential of halophytes in the development and rehabilitation of arid and semi-arid zones. In: Choukr-Allah R, Malcolm CV, Hamdy A (eds) Halophytes and biosaline agriculture. Marcel Dekker, New York, pp 3–13
Debez A, Ben Hamed K, Grignon C, Abdelly C (2004) Salinity effects on germination, growth, and seed production of the halophyte Cacile maritima. Plant Soil 262:179–189
Delesalle VA, Mazer SJ (1996) Nutrient levels and salinity affect gender and floral traits in the autogamous Spergularia marina. Int J Plant Sci 157:621–631
Desai BB, Kotecha PM, Salunkhe DK (1997) Seeds handbook. Marcel Dekker, New York
Dubey RS (1994) Protein synthesis by plants under stressful conditions. In: Passarakli M (ed) Handbook of plant and crop stress. MarcelDekker, New York, pp 277–299
Epstein E (1972) Mineral nutrition of plants: Principles and Perspectives. Wiley, New York
Fisher RA (1970) Statistical methods for research workers. Oliver & Boyd, Edinburgh
Flowers TJ (2004) Genetics of plant mineral nutrition. Improving crop salt tolerance. J Exp Bot 55:307–319
Gill PK, Sharma AD, Singh P, Bhullar SS (2001) Effect of various abiotic stresses on the growth. Soluble sugars and water relations of Sorghum seedlings grown in light and darkness. Bulg J Plant Physiol 27(1–2):72–84
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:149–190
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol 51:463–499
Hevia F, Wilckens R, Berti M, Badilla R (2001) Starch characteristics and protein content of quinoa (Chenopodium quinoa W.) grown under different nitrogen levels in Chillan. Agro sur 29(1):42–50
Hu YC, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
Jacobsen SE (2003) The worldwide potential of quinoa (Chenopodium quinoa Willd.). Food Rev Int 19:167–177
Jacobsen S-E (2007) Quinoa’s world potential. In: Ochatt S, Jain SM (eds) Breeding of neglected and under-utilized crops, spices and herbs. Science Publishers, Enfield, pp 109–122
Jacobsen SE, Quispe H, Mujica A (2001) Quinoa: an alternative crop for saline soils in the Andes. In: Scientist and Farmer-Partners in Research for the 21st Century. CIP Program Report 1999–2000, pp 403–408
Jacobsen SE, Mujica A, Jensen CR (2003) The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev Int 19:99–109
Jacobsen SE, Monteros C, Christiansen JL, Bravo LA, Corcuera LJ, Mujica A (2005) Plant responses of quinoa (Chenopodium quinoa Willd.) to frost at various phenological stages. Eur J Agron 22:131–139
Kermode AR (1990) Regulatory mechanisms involved in the transition from seed development to germination. Crit Rev Plant Sci 9:155–195
Kermode AR, Bewley JD (1985) The role of maturation drying in the transition from seed development to germination. I. Acquisition of dessication-tolerance and germinability during development of Ricinus communis L.seeds. J Exp Bot 36:1906–1915
Khan MA, Abdullah Z (2003) Salinity-sodicity induced changes in reproductive physiology of rice (Oryza sativa) under dense soil conditions. Environ Exp Bot 49(2):145–157
Khatun S, Flowers TJ (1995) Effect of salinity on seed set in rice. Plant Cell Environ 18:61–67
Konishi Y, Takezoe R, Murase J (1998) Energy dispersive X-ray microanalysis of element distribution in amaranth seed. Biosci Biotechnol Biochem 62:2288–2290
Konishi Y, Hirano S, Tsuboi H, Wada M (2004) Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci Biotechnol Biochem 68:231–234
Koyro H-W (2000) Effect of high NaCl-salinity on plant growth, leaf morphology, and ion composition in leaf tissues of Beta vulgaris ssp. maritima. J Appl Bot Food Qual 74:67–73
Koyro H-W (2003) Study of potential cash crop halophytes in a quick check system. Tasks Veg Sci 38:5–17 ISBN-4020-1202-0
Koyro H-W, Huchzermeyer B (1999) Salt and drought stress effects on metabolic regulation in maize. In: Pessarakli M (ed) Handbook of plant and crop stress. 2nd edn. Marcel Dekker, New York, pp 843–878
Koziol MJ (1992) Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J Food Compos Anal 5:35–68
Labidi N, Lachaal M, Soltani A, Grignon C, Hajji M (2004) Variability of the effects of salinity on reproductive capacity of Arabidopsis thaliana. J Plant Nutr 27:1561–1573
Levitt J (1980) Responses of plants to environmental stresses: water, radiation, salt, and other stresses. Academic, New York, pp 365–488
Lintschinger J, Fuchs N, Moser H, Jäger R, Hlebeina T, Markolin G, Gössler W (1997) Uptake of various trace elements during germination of wheat, buckwheat and quinoa. Plant Food Hum Nutr (Formerly Qualitas Plantarum) 50:223–237
Mahar AR, Hollington PA, Virk DS, Witcombe JR (2003) Selection for early heading and salt-tolerance in bread wheat. Cereal Res Commun 31:81–88
Marschner H, Kirkby EA, Cakmak I (1996) Effect of mineral nutrition status on shoot–root partitioning of photo-assimilates and cycling of mineral nutrients. J Exp Bot 49:1255–1263
Mujica A, Jacobsen SE, Ezquierdo J, Marathee JP (2001) Resultados de la Prueba Americana y Europes de la Quinua, FAO, UNA-Puno, p 51
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253:201–218
Munns R, Husain R, Rivelli AR, James RA, Condon AG, Lindsay MP, Lagudah ES, Schachtman DP, Hare RA (2002) Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil 247:93–105
Ng SC, Anderson AK (2005) Lipid oxidation in Quinoa (Chenopodium quinoa) determined through accelerated aging. Electron J Environ Agric Food Chem 4:1010–1020
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress Environments. Plant Physiol 109:735–742
Nrisingha D, Mandal RK (1993) Characterization of 2S albumin with nutritional balanced amino acid composition from the seeds of Chenopodium album and its antigenic homology with seed protein of some Chenopodium, Chenopodiaceae and Amaranthaceae species. Biochem Mol Biol Int 30:149–157
Passam HC, Kakouriotis D (1994) The effect of osmoconditioning on the germination, emergence and early plant growth of cucumber under saline conditions. Sci Hortic 57:233–240
Pitman MG, Läuchli A (2002) Global impact of salinity and agricultural ecosystems. In: Läuchli A, Lüttge U (eds) Salinity: environment – plants – molecules. Kluwer, Dordrecht, pp 3–20
Poustini K, Siosemardeh A (2004) Ion distribution in wheat cultivars in response to salinity stress. Field Crops Res 85:125–133
Prado FE, Boero C, Gallardo M, Gonzalez JA (2000) Effect of NaCl on germination, growth, and soluble sugar content in Chenopodium quinoa (Willd.) seeds. Bot Bull Acad Sin 41:27–34
Prakash D, Nath P, Pal M (1993) Composition, variation of nutritional contents in leaves, seed protein, fat and fatty acid profile of Chenopodium species. J Sci Food Agric 62:203–205
Prego I, Maldonado S, Otegui M (1998) Seed structure and localization of reserves in Chenopodium quinoa. Ann Bot 82:481–488
Puthur JT, Saradhi PP (2004) Developing embryos of Sesbania sesban have unique potential to photosynthesize under high osmotic environment. J Plant Physiol 161:1107–1118
Ranhotra GS, Gelroth JA, Glaser BK, Lorens KJ, Johnson DL (1993) Composition and nutritional quality of quinoa. Cereal Chem 70:303–305
Repo-Carrasco R, Espinoza C, Jacobsen SE (2003) Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev Int 19:179–189
Ruales J, Nair BM (1992) Nutritional quality of the protein in quinoa (Chenopodium quinoa Willd) seeds. Plant Food Hum Nutr 42:1–12
Ruales J, Nair BM (1993) Content of fat, vitamins and minerals in quinoa (Chenopodium quinoa Willd) seeds. Food Chem 48:131–136
Sild E, Pleijel H, Sellden G (2002) Elevated ozone (O3) alters carbohydrate metabolism during grain filling in wheat (Triticum aestivum L.). Agric Ecosyst Environ 92:71–81
Sivritepe HO, Dourado AM (1995) The effect of priming treatments on the viability and accumulation of chromosomal damage in aged pea seeds. Ann Bot 75:165–171
Sivritepe N, Sivritepe HO, Eris A (2003) The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci Hortic 97:229–237
Sosa L, Llanes A, Reinoso H, Reginato M, Luna V (2005) Osmotic and specific ion effects on the germination of Prosopis strombulifera. Ann Bot 96:261–267
Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42:211–220
Tanji KK (2002) Salinity in the soil environment. In: Läuchli A, Lüttge U (eds) Salinity: environment – plants – molecules. Kluwer, Dordrecht, pp 21–51
Thompson RD, Hueros G, Becker H, Maitz M (2001) Development and functions of seed transfer cells. Plant Sci 160(5):775–783
Thorne JT (1985) Phloem unloading of C and N assimilates in developing seeds. Ann Rev Plant Physiol 36:317–343
Ungar IA (1996) Effect of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae). Am J Bot 83:604–607
Varriano Marston E, DeFrancisco A (1984) Ultrastructure of quinoa fruit. Food Microstruct. 3:165–173
Walker DJ, Leigh RA, Miller AJ (1996) Potassium homeostasis in vacuolated plant cells. Proc Natl Acad Sci USA 93:10510–10514
Watanabe K, Ibuki A, Chen YC, Kawamura Y, Mitsunaga T (2003) Composition of quinoa protein fractions. J Jpn Soc Food Sci Technol 50(11):546–549
Weber H, Heim U, Golombek S, Borisjuk Land Wobus U (1998) Assimilate uptake and the regulation of seed development. Seed Sci Res 9:331–345
Weschke W, Panutz R, Sauer N, Wang Q, Neubohn W, Weber H, Wobus U (2000) Sucrose transport into barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J 21:455–467
Wild A (1999) Pflanzenphysiologische Versuche in der Schule. Quelle and Meyer Publisher, pp 413
Wilson HD (1990) Quinua and relatives (Chenopodium sect. Chenopodium subsect., Cellulata). Econ Bot 44:92–110
Wilson C, Read JJ, Abo-Kassem E (2002) Effect of mixed-salt salinity on growth and ion relations of a quinoa and a wheat variety. J Plant Nutr 25:2689–2704
Wood SG, Lawson LD, Fairbanks DJ, Robinson LR, Andersen WR (1993) Seed lipid content and fatty acid composition of three quinoa cultivars. J Food Compos Anal 6:41–44
Wyn Jones G, Gorham J (2002) Intra- and inter-cellular compartmentation of ions. In: Läuchli A, Lüttge U (eds) Salinity: environment–plants–molecules. Kluwer, Dordrecht, The Netherlands, pp 159–180
Xue Z-Y, Zhi D-Y, Xue GP, Zhang H, Zhao X-Y, Xia G-M (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Yeo A (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. J Exp Bot 49:915–929
Zhang H-X, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotech 19:765–768
Acknowledgements
The authors wish to thank Prof. Dr. Samy A. Habib, Ain Shams University, Faculty of Agriculture, Dept. of Agricultural Botany, Cairo, Egypt, Prof. Helmut Lieth, Institute of Environmental Systems Research (USF), University of Osnabrück, Germany and Dr. Nicole Geissler, Institute for Plant Ecology, University of Giessen, Germany for critically reading the manuscript. This work has been supported by a grant from the German Academic Exchange Service (DAAD, A/03/32801).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Rana E. Munns
Rights and permissions
About this article
Cite this article
Koyro, HW., Eisa, S.S. Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant Soil 302, 79–90 (2008). https://doi.org/10.1007/s11104-007-9457-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9457-4