Abstract
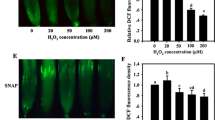
Rice (Oryza sativa L.) seedlings stressed with CdCl2 (0.5 mM or 50 μM) showed typical Cd toxicity (leaf chlorosis, decrease in chlorophyll content, or increase in H2O2 and malondialdehyde contents). Rice seedlings pretreated with heat shock at 45°C (HS) for 2 or 3 h were protected against subsequent Cd stress. Rice seedlings pretreated with HS had similar Cd concentration in leaves caused by CdCl2 as those non-HS. The content of H2O2 increased in leaves 1 h after HS exposure. However, APX and GR activities were higher in HS-treated leaves than their respective control, and it occurred after 2 h of HS treatment. Pretreatment of rice seedlings with H2O2 under non-HS conditions resulted in an increase in APX, GR, and CAT activities and protected rice seedlings from subsequent Cd stress. HS-induced H2O2 production and protection against subsequent Cd stress can be counteracted by imidazole, an inhibitor of NADPH oxidase complex. Results of the present study suggest that early accumulation of H2O2 during HS signals the increase in APX and GR activities, which in turn prevents rice seedlings from Cd-caused oxidative damage.









Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbate
- CAT:
-
Catalase
- DAB:
-
3, 3-Diaminobenzidine
- DW:
-
Dry weight
- GR:
-
Glutathione reductase
- HS:
-
Heat shock
- IMD:
-
Imidazole
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Alscher RG, Donahue JL, Cramer CK (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233
Azevedo Neto AD, Prisco JT, Enéas-Filho J, Medeiros J-VR, Gomes-Filho E (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol 162:114–1122
Badiani M, Paolacci AR, Fusari A, D’Ovidio R, Scandalios JG, Porceddu E, Sermanni G (1997) Non-optimal growth temperatures and antioxidants in the leaves of Sorghum bicolor (L.) Moench. II. Short-term acclimation. J Plant Physiol 151:409–421
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani EE (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Chen SL, Kao CH (1995a) Prior temperature exposure affects subsequent Cd-induced ethylene production in rice leaves. Plant Sci 104:135–138
Chen SL, Kao CH (1995b) Glutathione reduces the inhibition of rice seedling root growth caused by cadmium. Plant Growth Regul 16:249–252
Chen Z, Silva H, Klessig RF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262:1883–1886
Chien HF, Kao CH (2000) Accumulation of ammonium in rice leaves in response to excess cadmium. Plant Sci 156:111–115
Cho U-H, Seo N-H (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Das P, Samantaray S, Rout GR (1997) Studies on cadmium toxicity in plants: a review. Environ Pollut 98:2229–2236
Dat JF, Foyer CH, Scott IM (1998) Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol 118:1455–1461
Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J Exp Bot 52:1101–1109
Doke N (1997) The oxidative burst: roles in signal transduction and plant stress. In: Scandalios J (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 785–813
Foster JG, Hess JL (1980) Response of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associate mechanism of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Freeman JL, Persan MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thalspi nickel hyperaccumulators. Plant Cell 16:2129–2176
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Gong M, Chen B, Li X-G, Guo L-H (2001) Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J Plant Physiol 158:1125–1130
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hsu YT, Kao CH (2003) Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ 26:867–874
Hsu YT, Kao CH (2004) Cadmium toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Jana S, Choudhuri MH (1982) Glycolate metabolism of these submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Kang H-M, Saltveit ME (2001) Activity of enzyme antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiol Plant 1123:548556
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Kochhar S, Kochhar VK (2005) Expression of antioxidant enzymes and heat shock proteins in relation to combined stress of cadmium and heat in Vigna mungo seedldings. Plant Sci 168:921–929
Kocsy G, Von Ballmoss P, Suter M, Rüegseggger A, Galli U, Szalai G, Galiba G, Brunold C (2000) Inhibition of glutathione synthesis reduces chilling tolerance in maize. Planta 211:528–536
Kraus TE, Fleccher RA (1994) Paclobutrazol protects wheat seedlings from heat and paraquat injury: is detoxification of active oxgen involved? Plant Cell Physiol 35:45–52
Kuo MC, Kao CH (2004) Antioxidant enzyme activities are upregulated in response to cadmium in sensitive, but not in tolerant rice (Oryza sativa L.) seedlings. Bot Bull Acad Sin 45:291–299
Lopez-Delgado H, Dat JF, Foyer Ch, Scott IM (1998) Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J Exp Bot 49:713–720
Morita S, Kaminaka H, Masumura T, Tanaka C (1999) Induction of rice cytosolic ascorbte peroxidase mRNA by oxidative stress: involvement of hydrogen peroxide in oxidative stress signaling. Plant Cell Physiol 40:417–422
Nakano Y, Asda K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:807–880
Neumann D, Lichtenberger O, Günther D, Tschiersch K, Nover L (1994) Heat-shock proteins induce heavy-metal tolerance in higher plants. Planta 194:360–367
Nieto-Sotelo J, Ho T-HD (1986) Effect of heat shock on the metabolism of glutathione in maize roots. Plant Physiol 82:1031–1035
Noctror G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Olmos EO, Martínez-Solano JR, Piqueras A, Hellín E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54:291–301
Orozco-Cárdenas ML, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Orzech KA, Burke JJ (1988) Heat shock and the protection against metal toxicity in wheat leaves. Plant Cell Environ 11:711–714
Paoletti F, Aldinucci D, Mocali A, Capparini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541
Pei X, Murata Y, Benning G, Thomine S, Klusener B, Allen G, Grill E, Schroeder J (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734
Piqueras A, Olmos E, Martínez-Solano JR, Hellín E (1999) Cd induced oxidative burst in tobacco BY-2 cell: time-course, subcellular location and antioxidant response. Free Radic Res 31:S25–S31
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994a) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Prasad TK, Anderson MD, Stewart CR (1994b) Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol 105:619–627
Romero-Puertas MC, Zablza A, Rodriguez-Serrano M, Gómez M, del Río LA, Sandalio LM (2003) Antioxidative response to cadmium in pea roots. Free Radic Res 37:44
Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2004) Cadmium-induced subcellular accumultion of \(O^{ - }_{2} \) and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Sato Y, Murakami T, Funatsuki H, Matsube S, Saruyama H, Tanida M (2001) Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J Exp Bot 52:145–151
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shaw BP (1995) Effect of mercury and cadmium on the activities of antioxidative enzymes in the seedlings of Phaseolus aureus. Biol Plant 37:587–596
Susuki N, Koizumi N, Sano H (2001) Screening of cadmium-responsive genes in Arabidopsis thaliana. Plant Cell Environ 24:1177–1188
Tsai Y-C, Hong C-Y, Liu L-F, Kao CH (2004) Relative importance of Na+ and Cl− in NaCl-induced antioxidant sytems in roots of rice seedlings. Physiol Plant 122:86–94
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:5115–5523
Van Breusegem F, Vranová E, Dat JF, Inzé (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Volkov RA, Panchuk II, Mullineaux PM, Schöffl F (2006) Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61:733–746
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:83–294
Wintermans JFGM, De Mots A (1965) Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453
Xiang C, Werner BL, Christensen EM, Oliver DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126:564–574
Yu C-W, Murphy TM, Sung W-W, Lin C-H (2002) H2O2 treatment induced glutathione accumulation and chilling tolerance in mung bean. Funct Plant Biol 29:1081–1087
Yu C-W, Murphy TM, Lin C-H (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Acknowledgements
This work was supported by a research grant the National Science Council of the Republic of China (NSC 95-2313-B-002-046).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Hsu, Y.T., Kao, C.H. Heat shock-mediated H2O2 accumulation and protection against Cd toxicity in rice seedlings. Plant Soil 300, 137–147 (2007). https://doi.org/10.1007/s11104-007-9396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9396-0